The equilibrium constant, K, defines the relationship between the reactants and products of a reaction at equilibrium. A reaction reaches equilibrium when the rate of forwarding reaction and backward reaction is equal. Concentrations of all reactants and all the products are constant at equilibrium. A similar constant called reaction quotient Q is used when the reactants are not in equilibrium. It is equal to Kc.
What is Equilibrium Constant?
The equilibrium constant is the ratio of the concentration of products at equilibrium, raised to their stoichiometric coefficients, to the concentration of reactants at equilibrium, raised to their stoichiometric coefficients.
For example, a reversible reaction:
aA + bB = cC + dD
Then the equilibrium constant K is equal to:
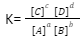
where,
[A] = Equilibrium Concentration of A
[B] = Equilibrium Concentration of B
[C] = Equilibrium Concentration of C
[D] = Equilibrium Concentration of D
For reactions that involve gases, the equilibrium constant is written in terms of the partial pressure of the gases.
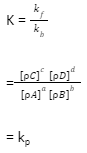
Here Kp defines the equilibrium constant in terms of partial pressures.
- Greater values of kc / kp indicate higher product formation and higher conversion percentages.
- Lower kc / kp values mean lower product formation and lower conversion percentages.
- Medium kc / kp values indicate optimum product formation.
Characteristics of Equilibrium Constant
Understanding the characteristics of equilibrium constant are important because they help calculate the equilibrium constant.
- The equilibrium constant has a definite value for each reaction at a given temperature.
- The equilibrium constant’s value is independent of the initial concentration of reactants.
- The presence of a catalyst does not affect the equilibrium constant. This is because the catalyst has an equal impact on the rate of forward and backward reactions.
- For the same reaction, the value of the equilibrium constant changes with the change in temperature.
- The equilibrium constant of reverse equilibrium is the reciprocal of the initial equilibrium i.e.
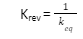
- Any change in the stoichiometry of the reaction leads to a change in the power of the equilibrium constant by the same quantity.
For example, if for the reaction A + B = C + D
the equilibrium constant is K
Then, for the reaction 3A + 3B = 3C + 3D
the equilibrium constant is K3
- In the case of stepwise multiple equilibria leading to the final products, the net equilibrium constant is equal to the product of each stepwise equilibrium constant. So, the net equilibrium constant K = K1 K2 K3
- Equilibrium reactions occur at the same time and produce the same product. The equilibrium constant of reactions remains constant. Product concentrations will be reduced due to the higher concentration of the common product.
Factors Affecting Equilibrium Constant
- The concentration of removed reactants or products is released by the reaction in the direction that replenishes the substance removed. When the concentration of the reactant or product changes, the composition of the mixture in chemical equilibrium changes.
- The change in volume causes the change in pressure. The total number of gaseous reactants and products change if the pressure changes, transforming the gaseous reaction.
- The rate of reaction is also affected by temperature changes. The equilibrium constant of an exothermic reaction decreases as temperature rises.
Calculating the Equilibrium Concentration
- The first step is substituting the values in the equilibrium constant formula.
- Next, determine the molar concentration or the partial pressures of the reactants and the products.
- Determine all equilibrium constant concentrations or partial pressure using the chart.
- Substitute into the equilibrium formula and solve for K.
For example:
A mixture of 0.100 M NO, 0.050 M H2, 0.100 M H2O was allowed to reach equilibrium (initially, the reaction didn’t have any N2). At equilibrium, the concentration of NO was found to be 0.062 M. Find the value of the equilibrium constant K for the reaction:
2 NO + 2 H2 = N2 + 2 H2O
Solution:
The reaction’s equilibrium expression is
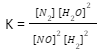
- Since Kc is being used, check to see if the amounts are expressed in moles per litre (molarity).
- Make an ICE chart for each species in the reaction that shows the initial concentration, the change in concentration, and the equilibrium concentration. The chart shows the changes in concentrations of each species as well as the equilibrium concentrations. We begin with the following information based on the example.
NO | H2 | N2 | H2O | |
Initial Concentration | 0.100 | 0.0500 | 0 | 0.100 |
Change in concentration | -2x | -2x | +x | +2x |
Equilibrium Concentration | 0.062 |
The difference in NO concentration was (0.062 M – 0.100M) = – 0.038 M. As a result, -2x = -0.038 and x = 0.019. It is important to note that the negative sign indicates a declining concentration, not a negative concentration.
NO | H2 | N2 | H2O | |
Initial Concentration | 0.100 | 0.0500 | 0 | 0.100 |
Change in concentration | -0.038 | -0.038 | +0.019 | +0.038 |
Equilibrium concentration | 0.062 | 0.012 | 0.019 | 0.138 |
Solve for Kc by substituting the equilibrium concentrations into the equilibrium expression.
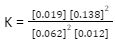
= 650 or 6.5 X 102
Conclusion
The equilibrium constant K defines the relationship between product and reactant in an equilibrium. Calculating the equilibrium concentration is easy if the values are given. The law of chemical equilibrium states that there is no change in the concentration of reactants or products after achieving a certain state, i.e. the equilibrium. It is possible to attain chemical equilibrium from either direction (forward or backwards). It is dynamic, which means the rates are equal without any change in the concentration of products and reactants.This equilibrium constant changes with changes in temperature, pressure and concentration.A catalyst can only increase or decrease the time of attaining the equilibrium state without any change in its concentration. Moreover, if the concentration is decreased, the reactants change the balance and go backwards. On the other hand, if the product concentration increases, then the equilibrium shifts towards the backward position, and if it decreases, it goes towards the forward path.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





