Atomic structure
Along with J.J. Thompson, there were other scientists such as Rutherford, Bohr, Dalton and Planck who formulated theories and explanations about the shape of an atom.
Thompson was the first scientist to share his knowledge about the atomic structure. He defined how the atom appeared like a watermelon in which the crimson, fleshy part was the illustration of protons, which are the positively charged particles, and the seeds represented the electrons, which are the negatively charged particles.
However, his concept had some limitations due to which it was not explored further. Following this, Rutherford came forward with his ideologies on atomic shape, for which he additionally explained the presence of neutrons via his experiment of gold foil.
Later on, Bohr, Dalton and Planck’s theories were also put forward to add to common knowledge about the atomic structure.
Now, the modern atomic structure that we study tells us that the atom consists of electrons, neutrons and protons in which the electron is the negatively charged species and the proton is the positively charged species. However, the neutron does not have any charge; it is known as an impartial element.
Along with this, there are necessary factors to be taken into consideration, which is that the proton and the neutron make up a group and consist of a nucleus inside the atom, whereas the electrons are present in orbits and rotate around the nucleus.
This is a representation of the structure of an atom:
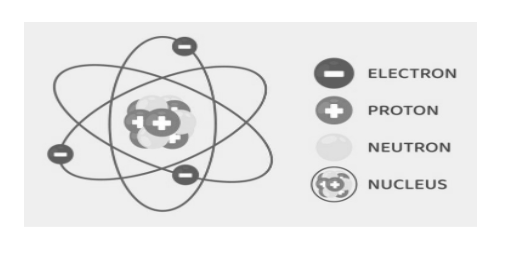
Subatomic particles
As cited previously, the atom essentially consists of three subatomic particles: electrons, protons, and neutrons.
Electrons: These are the negatively charged particles that rotate around the nucleus. They are also responsible for the formation of bonds when two elements come together in nature to form a balanced bond among themselves.
The quantity of electrons definitely present in any element also helps us determine the atomic number of the respective element.
For example, let’s look at hydrogen: it has only 1 electron, which revolves around the nucleus. Consequently, the atomic number of hydrogen will be 1.
Proton: Protons are the positively charged particles in an atom. They exist inside the nucleus at the centre of the atom, and act as an attractive force for the electrons, pulling them in the direction of the core.
They are also responsible for the centripetal force that acts in the direction of the nucleus from the outermost shell. Protons are useful for understanding the atomic mass of the species, along with the neutrons.
For example, if we want to discover the mass of any element, we will have to take into consideration the quantity of protons and the variety of neutrons in the respective element. If we add these values, we will get the final value of the mass of the element.
Neutron: Neutrons exist inside the nucleus in the atomic structure and do not have any charge, which means they are neutral in state. Therefore, they do not take part in the attracting and repelling movements that occur inside the atomic structure.
Neutrons are also useful in determining the atomic mass as it is known that the sum of protons and nucleons makes up the value of mass of any element. In some specific cases of isotope formation of any element, the variety of neutrons can fluctuate among them, which gives them their characteristic features.
Atomic & Mass Numbers of certain elements:
Hydrogen
Atomic number = 1 (one electron)
Mass number = 1 (one proton, no neutron)
Carbon
Atomic number = 6 (six electrons)
Mass number = 12 (6 protons and 6 neutrons)
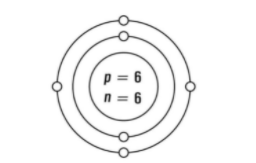
It has 2 shells around the nucleus in which the first shell present at the inner side has 2 electrons and the shell on the outer side has 4 electrons.
Oxygen
Atomic number = 8 (8 electrons)
Mass number = 16 (8 protons and 8 neutrons)
Nitrogen
Atomic number = 7
Mass number = 14 (7 protons and 7 neutrons)
Xenon
Atomic number = 54 (54 electrons)
Mass number = 131.29
Conclusion
The atom consists of three subatomic particles called neutrons, protons, and electrons. The number of electrons gives a representation of the atomic number, whereas the number of protons and neutrons give information about the atomic mass.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




