Atomic And Molecular Masses
Introduction
An atom is so small that it cannot be seen or separated. So, determining its real mass by weighing it is impossible.
Avogadro’s law, also known as Avogadro’s principle or Avogadro’s hypothesis, is a gas law which states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
Avogadro’s law is closely related to the ideal gas equation since it links temperature, pressure, volume, and amount of substance for a given gas.
Formula and Graphical Representation
At constant pressure and temperature, Avogadro’s law can be expressed via the following formula:
V ∝ n
V/n = k
Where V is the volume of the gas, n denotes the amount of gaseous substance (often expressed in moles), and k is a constant. When the amount of gaseous substance is increased, the corresponding increase in the volume occupied by the gas can be calculated with the help of the following formula:
V1/n1 = V2/n2 ( = k, as per Avogadro’s law).
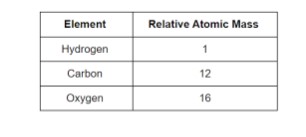
Although the atoms’ true masses can not be confirmed, their relative masses can be determined. The relative atomic mass of oxygen is 16 if the atomic mass of hydrogen is 1.
Initially, the atomic masses of all the elements were calculated by comparing them to the mass of hydrogen, which was assumed to be one, but this resulted in fractional atomic masses for the majority of the elements.
As a result, carbon is used as a standard for determining atomic masses.
Body
- Atomic Mass
It is the mass of an atom.
An element’s atomic mass is the number of times a molecule of that element is heavier than a carbon atom, which is 12. One atomic mass unit is one-twelfth of the mass of a carbon 12 isotope particle. When compared to a molecule of carbon 12 regarded as 12, the atomic mass of an element is the normal relative mass of its particles.
- The atomic mass unit (u; some texts use amu, but this is no longer recognised) is one-twelfth of the mass of a carbon-12 atom, a carbon isotope with six protons and six neutrons in its nucleus. A proton’s mass is 1.00728 u, a neutron’s mass is 1.00866 u, and an electron’s mass is 0.000549 u on this scale. If you count the total number of protons and neutrons in the nucleus (i.e., determine its mass number) and disregard the electrons, you can estimate an atom’s mass with a little mistake. Carbon-12 has a mass of about 12 u, oxygen-16 has a mass of about 16 u, and uranium-238 has a mass of about 238 u.
How to Work Out the Atomic Mass?
The atomic mass may be calculated using three distinct approaches.
- As a guide, use the periodic table:
An atomic number is normally given under the depiction of an element in the periodic table.
Consider the following scenario:
The atomic number of chlorine is 17, and its mass is 35.5.
The atomic number of chlorine is 20, and its mass is 40
In general, though, an atom’s atomic mass and its mass number will be relatively comparable, albeit the decimal places will vary.
- Protons and neutrons are added together.
- The average atomic mass of different elements is calculated by multiplying each isotope’s atomic mass by its fractional abundance and adding the result. Chlorine, for example, has two sorts of atoms with relative weights of 35u and 37u. In nature, these isotopes are found in a 3:1 ratio of relative abundance. As a result, the atomic mass of chlorine equals the sum of these several relative masses.
As a result, the average atomic mass of chlorine is calculated as
{(35 x 3) + (37 x 1)} / 4 = 35.5u
- Relative Atomic Mass
The relative atomic mass of an element is the relationship between its mass and the number of atoms it contains. The relative atomic mass scale is used to calculate the masses of different atoms.
The hydrogen atom, the lightest atom, was given a relative atomic mass of 1, and the relative atomic masses of other atoms were calculated in comparison.
- Gram Atomic Masses
The gram atomic masses of elements are their atomic masses given in gram. The atomic mass of an oxygen molecule, for example, is 16 amu.
As a result, oxygen has a gram atomic mass of 16 g.
- Molecular Mass
The number of times a material’s particle is heavier than one-twelfth the mass of a carbon atom – 12 – is the sub-atomic mass of the substance. Alternatively, the sub-atomic mass is equal to the sum of the atomic masses of the seeming plurality of particles present in a single material particle. Take, for example, water.
H has an atomic mass of one unit.
O has an atomic mass of 16 units.
Water has a subatomic mass of 2 atomic masses of H + 1 atomic mass of O.
= 2 × 1 + 16 × 1
= 18 units
Molecules’ molecular masses can be manipulated in the following way:
- Mass Spectrometry: This method is often used to determine the mass of small compounds. Monoisotopic mass is used to account for this.
- Hydrodynamic Strategy– Houwink relations are used in a hydrodynamic strategy because this approach necessitates calculation. It is sometimes referred to as a relative atomic weight determination procedure.
- Static Light Scattering: The Zimm approach is used to determine the molecular weight from the amount of light scattered.
- Mass photometry: MP is a label-free, in-solution technique of determining the molecular mass of proteins, lipids, carbohydrates, and nucleic acids at the single-molecule level. Interferometric scattered light microscopy is the basis of the technology. The mass of the molecule is linearly proportional to the contrast from scattered light caused by a single binding event at the interface between the protein solution and the glass slide. This approach may also be used to identify protein oligomerization status, characterize complex macromolecular assemblies (ribosomes, GroEL, AAV), and measure protein interactions such as protein-protein interactions.
Gram Molecular Mass
The gram sub-atomic mass is the sub-atomic mass of a material represented in grams.
Consider the following example: Oxygen has a molecular mass of 32u.
The subatomic mass of oxygen is 32 grams per gram.
- Formula Mass
Sodium chloride does not have separate molecules as component units.
For example, sodium chloride (NaCl).
Positive (sodium) and negative (chloride) entities are grouped in a three-dimensional structure in such compounds.
For example, the mass of sodium chloride (NaCl) is equal to the sum of the atomic masses of sodium (Na) and chlorine (Cl): 23.0 u + 35.5 u = 58.5 u.
Conclusion
- The mass of individual atoms and molecules is described by the atomic mass unit (u).
- The atomic mass of an element is the weight average of the masses of all its isotopes.
- The total of the masses of the atoms in a molecule is the molecular mass.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





