Introduction
The organic compounds, which are obtained by replacing one or more hydrogen atoms with one or more hydroxyl groups (-OH) linked to saturated carbon atoms, are called Alcohols. For example, methyl alcohol (methanol) and ethyl alcohol (ethanol) are two industrially important Alcohols.

Classification of Alcohols
They can further be categorized as-
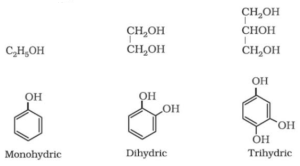
- Depending on the number of hydroxyl (-OH) groups present:
- Monohydric i.e., containing one -OH group
- Dihydric i.e., containing two -OH group
- Trihydric i.e., containing three -OH group
- Polyhydric i.e., containing many -OH group
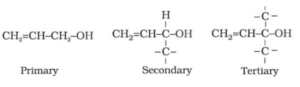
- Depending on the point of attachment of hydroxyl (-OH) group: Where the carbon atom of the alkyl group is attached to the -OH group.
-OH group attached at sp3 carbons:
a) Primary (10), Secondary (20), and Tertiary (30),

b) Allylic alcohol, the hydroxyl (-OH) group is linked to sp3 hybridized carbon, i.e., next to allylic carbon (i.e., the carbon-carbon double bond).
c) Benzylic alcohol, the hydroxyl (-OH) group is linked to the sp3 hybridized carbon atom which is next to an aromatic ring.
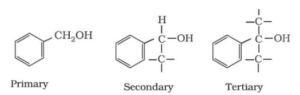
-OH group attached at sp2 carbons:
Vinylic alcohol, -OH group bonded to a carbon-carbon double bond (i.e., to a vinylic carbon or to an aryl carbon).
IUPAC System of Nomenclature
According to the IUPAC system of nomenclature, the alcohols are called Alkanols as the ‘-e’ of alkane in the derivative of corresponding alkanes are replaced by ‘-ol’.
Various steps in nomenclature are:
- Select the longest carbon chain which contains the carbon atom bearing -OH group. Identify the corresponding alkane and count the number of carbon atoms.
- Number the carbon chains beginning from the end closest to the hydroxyl (-OH) group.
- Number of other substituents according to their position on the chain.
- Write the name of alcohol by listing the substituents in the alphabetic order along with their position.
- The hydroxyl group takes precedence over double and triple bonds.
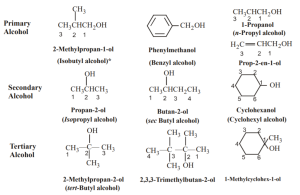
Some Common Alcohols and their names
Structure
- Structure of alcohol is similar to water. The sigma (σ) bond binds the carbon atom of the main chain to the oxygen atom of the hydroxyl group. The R-O-H bond angle is generally greater than the H-O-H bond angle in water as the alkyl groups are bulkier than the hydrogen atoms.
- The C-O-H bond angle is 108.90, which is less than tetrahedral and is larger than the hydrogen atom of water.
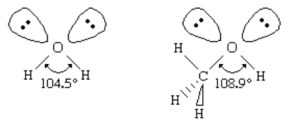
- In Methyl alcohol (Methanol), the C-O bond length is 142 pm.
Physical Properties of Alcohol
- COLOR: Mostly the alcohols are colorless liquids at room temperature.
- Methanol, ethanol, and isopropanol are free-flowing liquids and have a fruity odor.
- The alcohols which contain 4 to 10 carbon atoms are slightly viscous, oily, and have a fruity odor.
- Highly branched alcohols with more than 12 carbon atoms remain solid at room temperature.
- BOILING POINT: The boiling point increases with the increase in the number of C atoms, due to the increase in Van der Waals forces. The boiling point also decreases with an increase in the chain due to the decrease in surface area and Van der Waals forces. The boiling point of alcohol is more compared to other compounds like alkanes, ethers, haloalkanes, etc. due to intermolecular hydrogen bonding.
- SOLUBILITY: The solubility occurs due to the formation of hydrogen bonds with water. The solubility also decreases with the increase in the size of the alkyl group (hydrophobic in nature).
- ACIDITY: The acidity is because of the polarity of the O-H bond. The electron which releases alkyl groups has lower acidity. Hence, the order of acidity of alcohol is:
1° > 2° > 3°
H2O is a better proton donor or stronger acid than alcohol.
ALCOHOL CONSUMPTION
- Alcohol is a hallucinogenic substance with dependence-producing properties.
- The harmful use of alcohol leads to many diseases, along with the social and economic burden in societies.
- The harmful use of alcohol can also cause harm to family members, friends, co-workers and strangers.
- Alcohol consumption is the main causal factor for more than 200 diseases such as
- mental and behavioral disorders,
- alcohol dependence,
- noncommunicable diseases such as liver cirrhosis,
- some cancers and
- cardiovascular diseases,
- injuries resulting from collision, violence or road clashes,
- Alcohol consumption by pregnant women may suffer fetal alcohol syndrome and preterm birth complications.
Factors affecting alcohol consumption and alcohol-related harm
- Environmental factors include economic development,
- culture,
- availability of alcohol.
Alcohol Metabolism
The first step in the body used to break down alcohol is Alcohol dehydrogenase. Ethanol is highly toxic to the body and is found in alcoholic drinks.
Alcohol dehydrogenase is a zinc-based enzyme leading to the conversion of ethanol alcohol into acetaldehyde. Alcohol dehydrogenase is located in the cytosol of liver and stomach cells. The main enzyme for alcohol metabolism is ADH.
Enzyme Mechanism
The general mechanism shows that the ethanol + NAD forms acetaldehyde + NADH. In other words, ethanol (it is any carbon chain with carbon attached to an OH group) is oxidized into acetaldehyde (it is any carbon chain with the terminal carbon forming a double bond with oxygen) and the hydrogen atoms are added to NAD.
The active site of alcohol dehydrogenase includes a serine, histidine, isoleucine and zinc stabilized by two cysteines and a histidine.
Zinc binds to the oxygen in the alcohol. The NAD gets bound to the isoleucine. The two main steps in the alcohol hydrogenase mechanism are:
- Hydrogen is removed from the -OH on the alcohol.
- Hydrogen is removed from the carbon and added to NAD to form NADH leading to the formation of a carbon-oxygen double bond.
In step 1, histidine removes a hydrogen atom from NAD, and then it removes hydrogen from serine as a result to which it takes the hydrogen from the OH on the alcohol.
In step 2, a double bond is formed due to the presence of electrons in the oxygen with carbon. To the 6-membered ring on NAD, the electrons in the carbon-hydrogen bond are added (with the hydrogen) leading to the formation of NADH and acetaldehyde.
USE OF ALCOHOL
METHANOL:
a) It is used in automotive radiators as an anti-freeze.
b) It is used as a fuel for internal combustion in engines.
c) It is used as an additive in petrol to improve combustion.
d) It is used as a solvent in inks, dyes, resins, etc.
e) It is also used in the pharmaceutical industry.
f) It is used as a chemical feedstock.
g) Methanol is used in the manufacturing of paints, plastics, cosmetics, textiles, etc.
ETHANOL:
a) Ethanol is considered an alcoholic drink. Its content is present in beers and wines.
b) It is used as transportation fuel or is mixed with petrol as it burns with smokeless clean flame by undergoing complete combustion.
c) It is used in paints, cosmetics, inks, perfumes, etc.
d) It is also used as a solvent.
e) Any organic compound which is not soluble in water gets easily dissolved in ethanol.
f) It has antiseptic properties.
g) It slows or stops the growth of microorganisms by denaturation process, hence, is used in sanitisers and hand wipes.
PROPANOL:
a) It is used as a solvent.
b) It is also used in the pharmaceutical industry.
c) It also has antiseptic properties.
BUTANOL:
a) Its use is increased as a biofuel which is produced from the fermentation of sugar and carbohydrates.
b) It is used as a solvent in perfumes, paints, cosmetics, inks, etc.
c) Butanol is used in manufacturing butyl ethanoate, an ester. It is also used as a synthetic fruit flavor which is used in the confectionery and food industry.
Conclusion
We have seen how alcohols are molecules that include one or more hydroxyl (-OH) groups directly connected to a carbon chain, as opposed to other compounds. Generally speaking, alcohols in the free-form are not found in nature; instead, they are found mostly in the essential or volatile oils extracted from the flowers, leaves, and stems of various plants.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





