The Van der Waals equation describes the connection between pressure, volume, temperature, and number of genuine gasses. The equation for a real gas with ‘n’ moles is as per the following:
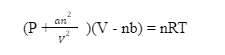
The tension, volume, temperature, and moles of the gas are addressed as P, V, T, n. Constants ‘a’ and ‘b’ that are interesting to each gas.
The Units of Vander Waals equation:
In the van der Waals equation, there are two rectification factors. In the best gas equation, the first an²⁄ V², adjusts the strain. The intermolecular alluring interactions between gas atoms are
represented. The strength of the intermolecular alluring power is indicated by the size of a. and is composed of units . (L² atm) ⁄ mol²
The volume involved by the gas atoms is addressed by the component – nb. The units of b are
L ⁄ mol. Because b addresses the general volume per mole filled by gas atoms, it is basically the same as the volume per mole of the fluid state, which has firmly stacked particles. The extent of b is generally altogether more modest than that of a. With the size and intricacy of the particle, the upsides of an and b will quite often rise.
For the calculation of units and aspects of constants a and b, the Van der Waals equation is used. an² ⁄ V² = is the Van der Waals equation for n-mole real gasses, which is the unit of internal tension. Subsequently, the Van der Waals constant is estimated in, a= atm lit² mol-² . Again, nb = volume unit, therefore unit of Van der Waals constant, and b = lit mol-¹ .
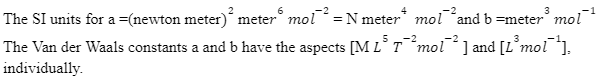
Vendor was constant:
The van der Waals equation is a state equation that records for two parts of real gases: gas molecule prohibited volume and gas atom appealing powers.
The van der Waals equation is usually expressed as follows:
(P+an² ⁄ V²)(V-nb) = nrt
The constants a and b are gas-explicit and describe the greatness of intermolecular fascination and rejected volume, individually.
Van der Waals constant significance:
1.The worth of the tension amendment factor
The intermolecular fascination causes the tension rectification factor (a) = an² ⁄ V², and therefore,
the strain remedy component can be utilized to determine the gas’ internal tension. Nonetheless, the more prominent the worth of the strain revision factor, the more grounded the intermolecular fascination and the simpler the gas will melt. Subsequently, the Van der Waals constant (a) for carbon dioxide gas is 3.95, but hydrogen gas is 0.2.
2.The worth of the volume amendment factor
The particle size as well as frightful powers are estimated by another constant volume rectification factor (b). The bigger the gas atom, the higher the worth of b. Therefore, the Van der Waals constant b for carbon dioxide is 0.04 while it is 0.02 for hydrogen.
3.Van der Waals gases
At 0°C, appealing powers dominated the behavior of Van der Waals gasses (carbon dioxide, nitrogen, and hydrocarbons like methane, ethane, and acetylene), but the sub-atomic size impact dominated the behavior of hydrogen gas. Because these gasses are challenging to condense, the unit worth of Van der Waals constant hydrogen, helium, nitrogen, and other gasses utilized in science and physical science is tiny.
Unit of b in real gas equation:
A gas that doesn’t carry on like an ideal gas is alluded to as a “real gas.” The interactions between the gaseous atoms assist with explaining their behavior. Real gasses don’t keep the ideal gas regulation because of these intermolecular interactions between gas particles.
Therefore, Real gasses are non-ideal gasses with particles that take up a particular measure of room and can interact with each other.
A few components should be examined in request to appreciate how real gasses behave. The different contemplations that should be made while dealing with genuine gasses are outlined below.
Ø Impacts of compressibility on the real gas
Ø Different real gasses have different explicit hotness limits.
Ø Van der Waals powers affect the interactions between atoms in a real gas.
Ø The framework’s true capacity for non-equilibrium thermodynamic impacts.
Ø The gas’ different organization and changes in structure because of atomic separation, as well as any rudimentary cycles that might happen.
Positive qualities are allotted to the constants a and b, which are special to each gas. Intermolecular fascination is revised by the term involving the constant a. Alluring powers between atoms bring down the strain of a real gas, causing particles to dial back and decrease impacts with the dividers.
The more prominent the fascination between atoms and the more straightforward the gas packs, the higher the worth of a. The prohibited volume of the gas, or the volume involved by the gas particles, is addressed by the b term. The units of b are L ⁄ mol . Because b addresses the general
volume per mole filled by gas atoms
CONCLUSION:
For a real gas, the van der Waals equation of state is:
(P+an² ⁄ V²)(V-nb) = nrt
P addresses pressure, V addresses volume, T addresses temperature, n addresses how much substance (in moles), and R addresses the gas constant. The van der Waals constants a and b are properties of the substance and are temperature independent.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













