The terms molarity and molality describe a chemical solution’s concentration. The relation between molarity and molality is the two boils down to the difference between mass and volume. The molality of a solute is referred to as the number of moles of a solute with the mass of a solvent. In contrast, the molarity of a solute is defined as the number of moles of a solute in proportion to the volume of a solution. Please continue reading to learn more about the relation between molarity and molality and their meanings, calculations, and comparing the two concepts.
What is Molality?
It is measured in molality (m), also known as molal concentration, the quantity of a material dissolved in a given mass of a solvent. It is defined as the number of moles of a solute per kilogramme of a solvent in a certain amount of solvent.
The formula for molality and molality units
Molality is measured in milligrammes (m) or milligrammes per kilogramme (mol/kg).
The molality equation is a mathematical formula that describes how much a substance weighs.
In this equation, m = moles solute per kilogramme of solution
What is the definition of molarity?
Molarity (M) is the quantity of a material in a specific volume of solution. Molarity can be defined as the moles of a solute per litres of a solution. Molarity is also known as the molar concentration of a solution.
Molarity formula and units
The units of molarity are M or mol/L. A 1 M solution is indicated as a “one molar solution.”
Molarity equation- M is equal to moles of solute per litre of solution.
What is the relation between molarity and molality?
The composition of a solution is described by the word ‘Concentration’. Now, it depends on us whether we characterise it numerically or qualitatively. Like if we characterise it qualitatively, we would say that the solution is either dilute (less quantity of solute is present) or concentrated (greater quantity of solute is present) (more quantity of solute is present).
But there’s a challenge in defining the concentration of a solution qualitatively, such as what should be the lowest quantity of solute for the solution to be termed the concentrated or maximum amount of solute present in the solution for it to be considered dilute.
So, in order to address this problem, we have to define concentration quantitatively. For example, molarity and molality are two concentration concepts.
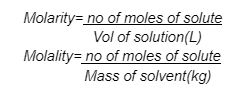
Molality’s Characteristic
A solution’s molality is defined as one of its properties. It will not differ from one sample to the next because it is an intensity feature.
The number of moles of solute or the mass of solvent is unaffected by temperature or pressure. Because molality is not a molar quantity, it is unaffected by temperature or pressure.
Molality refers to a change in concentration that isn’t defined in terms of volume, such as mass or mole fractions.
Molality vs Molarity: What’s the Difference?
Molality refers to the mass of the solvent, whereas molarity refers to the total volume of the solution.
Molality is denoted by the lower case “m,” while Molarity is denoted by the upper case “M.”
Molality is measured in moles per kilogramme (moles/kg), whereas Molarity is measured in moles per litre (moles/litre).
What are the advantages and disadvantages?
Advantages
- The molality of one solute in a solution is freed in the presence or absence of another solute, which is another advantage of molality.
- One of the most important characteristics of a solution is its molality. It is used to express the concentration of a solute in a solution and is mostly determined by the solvent’s mass.
Disadvantages
- It’s useless for cases when there’s no pure essence in the mix.
- A blend of water and liquor, for example, permits the norm to be followed. Any chemical can be used as a solvent in this case.
Conclusion
In a chemical solution, the molarity and molality both serve as indicators of its concentration. There is a difference between molality (the number of moles per kilogramme of solvent) and the quantity measured by moles per litre of solution (mol/L). Most of the time, it doesn’t matter whatsoever which unit of concentration you use. However, molality is recommended when a solution will undergo temperature fluctuations as adjusting temperature impacts volume (thus changing the concentration if molarity is utilised) (thus changing the concentration if molarity is used) (thus changing the concentration if molarity is used). Above, we have briefly mentioned the relation between molarity and molality.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out









