Equilibrium is a state or condition at which the rate of the forward reaction is similar to the rate of backward reaction for any reversible reaction, the macroscopic properties, and the system remains in stable change after the reaction occurs and does not change. Suppose there is a container that contains water, now when the container is heated the water which is in the liquid state will change into a vapor state, if the container is closed from the top, then the water condenses and becomes water again. So, this is a reversible reaction and is equilibrium in nature. Here the forward reaction is from liquid to vapor and the backward reaction from vapor to liquid is in an equilibrium state.
Ionic Equilibrium
Ionic Equilibrium in solutions
Ionic equilibrium is an equilibrium state in which there is a balance in the solution between ions and the unionized molecule. For example, breaking of acetic acid into hydrogen ions and acetate ions
CH3 COOH ⇄CH3 COO- + H+
Electrolysis is a kind of state where the electricity can be conducted by chemical substances in their molten state or aqueous state. Due to the ionic movement, the current is conducted through electrolytes. At a particular temperature, the product of the concentration of hydroxyl ions and hydrogen ions is constant. It is called the product of water or ions and is denoted as Kw. By observing the autoionization reaction the idea can be gathered of the ionic product of water. This is expressed as:
H2O + H2O = H3O+ + OH–
Here,
Kw = [H3 O+ ][OH–]
At 25℃ the value of Kw = 1 X 10-14
In the aqueous solution the study that deals with the acid-base Equilibria, the primary interest of all is in the solution’s hydrogen ion concentration. These are the dilute solutions of which it is to be dealt with, the concentration of hydrogen ion is something to the power -10.
The measurement of hydrogen ion concentration is the pH. It is the measurement of the alkalinity or acidity of a solution. At 25℃ The acidic aqueous solutions have a pH of less than seven and those aqueous solutions which are alkaline or basic have a pH of more than seven.
the pH of a given solution can be calculated using the formula:
pH = -log [H+]
Strong electrolytes are the types of electrolytes that fully dissociate in constituent ions of the aqueous solution. For example, mineral acids such as H2SO4, HCl, HNO3, etc.
The weak electrolytes are the type of electrolytes that to a lower extent dissociate in the aqueous solution. Weak electrolytes are all organic acids (leaving the sulphonic acids) and bases include amines and NH4.NH4OH, etc., all types of salts except CdBr2 and HgCl2, and some bases like NaOH, KOH, etc.
Sparingly Soluble Salts
Sparingly soluble salts are those salts which are having less solubility in water at simple temperatures. One example of sparingly soluble salt is silver chloride. When in the water a large amount of sparingly soluble salt is dissolved, then a part of the salt goes to the solution and becomes saturated at normal room temperature. It seems that the process of dissolution has stopped. But between the ions in the solution and the solid salt forms a dynamic equilibrium. It can be expressed as:
AgCl (solid) → Ag+ + Cl–
At any equilibrium reaction, here, like Ionic equilibrium, the forward reaction is equal or equivalent to the backward reaction.
Ionization degree or Dissociation degree (α)
The ionization degree or dissociation degree is the division of molecules of a total number that dissociates into component ions.
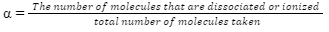
In the case of strong electrolytes, α = 1
In the case of weak electrolytes, α < 1
Dilution Law of Ostwald
The square root of the solution’s molar concentration is disproportionate to the degree of ionization of electrolytes which are weak in nature.
K = Cα2/ 1 – α
If α is very small
Then,
1 – α 1 => Ka = Cα2
- α = √Kα / C
- α 1 / √C
Here,
K = Dissociation Constant
C = Solution’s molar concentration
Conclusion
It is concluded that Ionic equilibrium is an equilibrium state in which there is a balance in the solution between ions and the unionized molecule. The equilibrium state is defined as when a reaction has both forward and backward reactions, that is the reaction is reversible. There are two types of equilibrium: physical equilibrium and chemical equilibrium.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













