Nitration is the process of incorporation of nitro group (NO2) in the organic aliphatic or aromatic compounds. Nitration mechanism is used in various synthesis like in the production of nitrobenzene, for producing explosives [for example; toluene is converted into trinitrotoluene (TNT)]. It is also used as chemical precursors or intermediaries. Basically, the replacement of organic compound hydrogen with the nitro group is also called nitration mechanism which usually takes place at high temperature and is referred to as the exothermic reaction.
In 1971, Skinner and Perrin first used the term ipso nitration during investigation of nitration of chloroanisole. The conversion of functional groups like ethyl, hydroxyl, carboxyl, halogens and many others which are attached or linked to either aromatic rings or aliphatic chains into the nitro group in the mixture of nitration is known as ipso nitration. In the protocol of nitration 4-chloro-n-butylbenzene react with the compound sodium nitrite in the presence of PTC (phase transfer catalyst) as well as ligand of 0.5% biaryl phosphine for the formation of 4-nitro-n-butylbenzene compound.
The main difference between ordinary nitration mechanism and ipso nitration mechanism is that ordinary nitration is the simple introduction of nitro group in the place of hydrogen while ipso nitration is the substitution of functional groups like propyl, hydroxyl etc. with the nitro group (-NO2). Initially the ipso nitration is developed by either HNO3 (nitric acid) use or their nitration mixtures like HNO3/H2SO4 which is now regarded as a classical or traditional method of preparing ipso subsctituted products. Several developments had been done for developing new products of nitrogen compounds either by decreasing or increasing HNO3 (nitric acid) levels or by using non-catalytic or catalytic methods i.e. use of catalyst.
Catalyst meaning: Catalyst is the compound or the substance which initiates or enhances the chemical reaction.
The example of ipso nitration can be represented as;
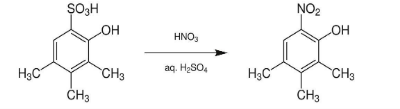
Ipso Nitration of Aryl Boronic Acid with Fumic Nitric Acid and Chlorotrimethylsilane
Ipso Nitration of Aryl Boronic Acid with Fumic Nitric Acid
This procedure provides chemoselectivity as well as an efficient approach to various different varieties of nitro aromatic compounds. This reaction takes place in the presence of fumic nitric acid and will convert the aryl boronic acid into its aryl nitrogen derivative.
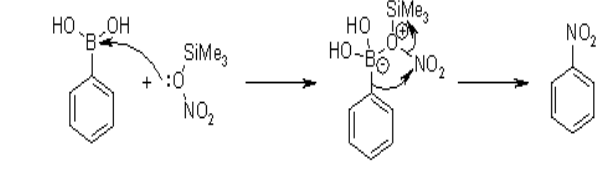
Ipso Nitration of Aryl Boronic Acid with Chlorotrimethylsilane
The nitrating agent in this reaction of aryl boronic acid is the mixture of chlorotrimethylsilane as well as the nitrate salt for production of excellent and efficient yields of nitroarenes. This reaction is highly convenient, selective and simple and also provides a product with greater yield. This reaction can be represented as;
Recent Developments and Modern Approaches in Ipso Nitration
The developments in the classical and traditional method of ipso nitration mechanism are as follows;
- Macromolecules Ipso Nitration (calixarenes).
- Heterocycles Ipso Nitration.
- Use of CAN (Cerium Ammonium Nitrate) as the nitrating agent.
The modern approaches to ipso nitration are as follows;
- The carboxyl group’s ipso nitration.
- Halogens ipso nitration.
- Arylboronic acid ipso nitration.
Applications of Nitration
The applications of nitration are as follows;
- The nitration products can be used as explosives, solvents, pharmaceuticals, dyestuffs and many more.
- Its mechanism is used in synthesis of various compounds like nitroglycerin and other nitroaromatic compounds.
- It is also used as intermediaries for the production of other organic compounds like amines.
- It works as a catalyst for several chemical reactions.
Conclusion
Ipso Nitration is the term coined in 1971 by Skinner and Perrin. This is the mechanism or reaction in which the functional group is replaced by the nitro group (-NO2 group) in the presence of the nitrating agent which works as a catalyst. For example; the aryl boronic acid is converted into the aryl derivatives of nitrogen in the presence of nitrating agents or catalysts like fumic nitric acid
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out








