Normality as a measure of concentration:
It is a well-known fact that in nature, any reaction takes place between particular proportions of reacting elements. This we know as the stoichiometry of a reaction. Another important fact about stoichiometric proportions is that it deals with several particles of an element, like atoms or molecules, and not with the masses of the components. Concentration measures like molarity, normality, molality, and formality express concentration with respect to several participating atoms or molecules of component elements or compounds.
For any solution, in general, normality is expressed as a number of gram equivalents per litre of a solution. Gram equivalent can be understood as a reacting functional unit of a compound. Thus normality gives importance to reacting species of a component to express its strength.
Mathematical expression for normality is given as:
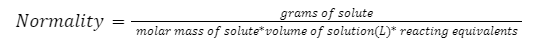
It is known that the number of moles of solute per litre of a solution is molarity. Thus the formula for normality can be reduced to:
Normality=Molarity ⁄ reacting equivalents
Normality is preferred mostly for acid-base solutions and sometimes for salt solutions. We will see how to calculate normality for both these types of solutions. Along with this, we will also learn how to calculate the required amount of solute to make a particular volume solution of given normality.
Normality for acid-base solutions:
An acid or a base reacting species are either hydronium ion (H+) or hydroxide ion (-OH), respectively. Thus normality of a solution will express the relative strength of an acidic or basic solution to lose hydronium ion or hydroxyl ion, respectively.
Thus, the formula for normality for acidic or a basic solution is:
Normality=molarity ⁄ number of Hydronium ions or hydroxide ions
Let’s take two examples,
Example1: what is the normality of 108.38gm of HCl making 1000ml of volume?
Solution: we will first calculate the molarity of a solution. The molar mass of an HCl is 36.46, so the molarity will be:
Molarity=weight in grammar ⁄ mass of solute*volume of solution in a litter
molarity of HCl=108.38 ⁄ 36.46*1=3M
For a hydrochloric acid, a number of reacting equivalents is one. So, with the above formula, the normality of the given solution will be:
Normality of HCl=3 ⁄ 1=3N
Example2: calculate normality for 1000ml of 87.478gm of magnesium hydroxide (Mg(OH)2).
Solution: As for the above example, first calculate molarity. The molecular mass of magnesium hydroxide is 58.319
molarity =87.478 ⁄ 58.319*1=1.5M
Magnesium hydroxide is a di-acidic base that means the reacting equivalent is two.
Normality =1.5 ⁄ 2=0.75N
Normality of salt solution:
For a salt solution, reacting equivalents are related to the valence number. In other terms, it is several hydrogen atoms required to displace a cation from the compound. Thus normality formula for a salt solution will be:
Normality=Molarity ⁄ valance number
Let’s take an example.
Example1: what will be the normality of a solution of 233.76gm of sodium chloride (volume 1 L)
Solution: molar mass of sodium chloride is 58.44 thus the molarity of the solution will be
molarity =233.76 ⁄ 58.44*1=4M
Now, the equivalent for sodium chloride will be one. So, the normality will be
Normality =4 ⁄ 1=4N
Calculating the required amount of solute from given normality:
Another type of question that comes based on normality concepts is we have to calculate the required quantity of a solute to make the particular volume of a solution with given normality. We will see one example of this type.
Example1: How many grams of sulphuric sodium acid will be required to make a one-litre solution of concentration 2N?
Solution: we know that sulphuric acid (H2SO4) is dibasic acid. That means its reacting equivalence is 2. So the required molarity of the solution to make a 1N solution of 1L volume will be:
Molarity=Normality*Number equivalent
Molarity=2*2=4M
Now, the molar mass of sulphuric acid is 98.08. Thus the required amount of sulphuric acid in gram to make a 1L solution of 4M concentration is:
A gram of solute=molarity*Volume in L*molar mass of solute
Gram of solute=4*1*98.08=392.32gm
Lastly, since sulphuric acid is in a liquid state, we will calculate the equivalent volume of sulphuric acid corresponding to 392.32gm. The density of sulphuric acid is 1.83gm/cm3.
Volume=weight ⁄ density
Volume=392.32 ⁄ 1.83=215.56cubic cm
Conclusion:
Normality is an important measure of concentration. It deals with the relative reacting equivalent strength of the solution. Knowing the normality of an acidic or basic solution is very important while working with an experiment that involves acidic or basic entities. Also, it is always important to use correct and accurate normal solutions to get proper results. Hence it is beneficial to practice several calculations regarding the normality of a solution. This will surely help in practical purposes, especially in the subject of chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













