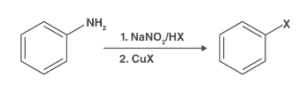
Sandmeyer reaction is the chemical process used to synthesize the aryl halides from the aryl diazonium salts. Copper is used as the catalyst in this process which aids in the substitution of the aromatic amino group through the preparation of the diazonium salts. Catalysts are famous for increasing the temperature of the reaction or increasing the activation energy of the reaction. This section will cover all about the reagent used in the Sandmeyer reaction.
It becomes easy for the students to understand the role of catalysts in organic reactions like Sandmeyer reactions. We’ll start with a quick definition of the Sandmeyer reaction, a Sandmeyer reaction example, etc., followed by the brief details of the reagent used in this reaction.
Sandmeyer reaction: A quick revision
It is an organic chemical reaction in which substitution takes place in the presence of copper catalysts. It has a radical-nucleophilic mechanism that uses the copper salts iodides, chlorides, or bromides.
Sandmeyer reaction takes place in two steps, including forming the diazonium salts and transforming diazo intermediates into aryl halides. The creation of diazonium salts takes place with the reaction of sodium nitrite and acid in the presence of copper salts. The diazonium ion thus formed gets converted into the aryl halides. It takes place in the presence of water, cyanide, and anions.
Sandmeyer reaction example
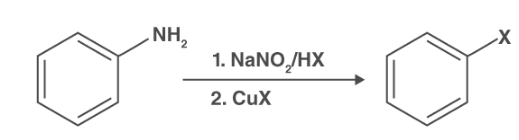
Why is copper used in the Sandmeyer reaction?
Sandmeyer reaction requires sodium nitrite and acid to create the nitrous acid, which helps form nitrosonium ion. The first-step protonation helps in the formation of nitrosonium ions. This ion works as the electrophile, which reacts with heterocyclic amine or aromatic amine. Here, the diazonium salt is formed in this step.
When it comes to the use of copper in the Sandmeyer reaction is credited with generating the copper halides. The diazo radical and copper halides are formed by transferring a single electron from the copper catalyst to the diazonium. Hence, the role of reagents is essential in the first step of the Sandmeyer reaction.
The N2 molecule is released in the next step in the Sandmeyer reaction from the diazo radical, forming the Aryl radical. This Aryl radical reacts with the copper halide to maintain the copper halide and create the stable aryl halide. Hence, the role of the copper catalyst is indispensable in this part of the Sandmeyer reaction.
Some of the critical applications of the Sandmeyer reaction using the reagents are:
- Hydroxylation- It is used to convert the arylamines to phenols and finally lead the way for the formation of aryl diazonium salts.
- Trifluoromethylation- It is used to create aryl compounds in the formation of trifluoromethylation. This process is further used in multiple practical applications.
- Cyanation- It is used to create Benzonitriles which are one of the widely used organic compounds. One of the widely used applications of the cyanation process is the creation of Fluanxol, which is a popular antipsychotic drug.
- Aryl halide synthesis- Different aryl halides like aryl iodides, aryl bromides, and aryl chlorides can be synthesized using the Sandmeyer reaction. Aryl iodides are synthesized using di-iodomethane, aryl bromides are synthesized using bromoform, and aryl chlorides are synthesized using chloroform.
Hence, any application of the Sandmeyer reaction is incomplete without the critical reagents which are used in the Sandmeyer reaction.
Conclusion
Catalysts are an essential part of the chemical reactions as these can significantly improve the rate of response and adds to the yield of reactions. Catalysts work by lowering the transition state while lowering the activation energy or by changing the reaction mechanism. Hence, multiple reagents are used in the Sandmeyer reaction.
It becomes easy for the students to understand the Sandmeyer reaction example with the help of copper catalysts. The use of copper in the Sandmeyer reaction significantly contributes to the completion of the process. Further, the quick questions related to this exclusive organic reaction help students go through the main doubts without referring to different study materials.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out