Water is counted as the essential resource for life. It is found in abundance on earth. A water molecule is made of hydrogen and oxygen. If we try to break a molecule of water, it will break into hydrogen and oxygen. We know that when an atom or molecule breaks, positive and negative both charges get liberated. Positive and negative charges attract each other thus making a substance. Ionization is a process in which a substance gets converted into ions. Whenever the phenomenon of ionization takes place, we get ions that readily react with other substances to form new products as suitable to their characteristics.
Body
The ionic theory was proposed by a famous scientist Svante Arrhenius. According to his theory, whenever an electrolyte is dissolved in any polarized solvent or in water, it gets divided into two kinds of charged particles, positive ions and negative ions; this process is called ionization. This process uses the electrolyte, that’s why it is known as the process of ‘electrolytic dissolution’. An atom which possesses an electric charge is called ‘ion’. Such ions can be negative or positive in nature.
In case of ionization of water, water gets divided into two ions-
One is positively charged hydrogen ion (H+) or hydronium ion (H3O+) and another one is negatively charged hydroxide ion (OH-).
H2O + H2O ⇌ H3O+ + OH-
We see here that two molecules of hydrogen produce two different ions in the process of ionization. It is the equation of ionization of water.
Ionization constant of Water-
To find the value of ionization of water, we divide the ions to molecules of water. We denote the ‘ionization constant of water’ by Kw. It is based on the concept that the solution of electrolyte is always neutral because the number of positive ions is always equal to negative ions. So, the equation of ionization of water –
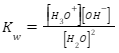
Value of ionization of water
We can find the concentration of a substance on the pH scale. In measurements, when we keep the temperature at 25° C, the concentration of hydronium ion is recorded 1×10-7 and the concentration of hydroxide ion is recorded 1×10-7. For pure water, the concentration is 1. If we put these values in our ‘equation of ionization of water’, we find-
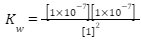
Degree of ionization
When we dissolve the electrolyte into water, the process of ionization doesn’t affect them all. It means that all the molecules don’t get ionized in the process of ionization. So, the part of molecules of the electrolyte which gets dissolved or gets ionized, is called the ‘degree of ionization’ or sometimes ‘degree of dissociation’ as ions are getting disassociated. Basically, the degree of ionization is the number of molecules which are ionized. We can express it as-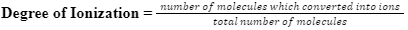
Obviously, its value will be less than 1 because the total number of molecules will always be greater than the number of molecules which become ions.
We can also find the percentage of ionization by the formula if we have the value of ‘degree of ionization’-
Percentage of Ionization = degree of ionization 100
Conclusion
One molecule of water is made of two ions. One is hydronium or hydrogen ion and another one is hydroxide ion. One is a positive ion and another one is a negative ion, that’s why water Itself has no charge as both the charges neutralize each other. In the process of ionization, we can see both the charges of water separately. We can find how many molecules are ionized and how many are not ionized during the process of ionization, by the equation of ionization of water.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













