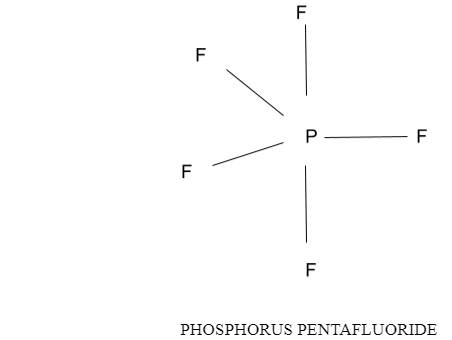
Repulsion between the five sets of valence electrons on the phosphorus molecule in PF5 can be limited by appropriating these electrons toward the sides of a three-sided bipyramid. Three of the situations in a three-sided bipyramid are marked central since they lie along the equator of the particle. The other two are pivotal on the grounds that they lie along a pivot opposite to the central plane. The point between the three central positions is 120 while the point between a pivotal and a tropical position is 90.
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) hypothesis, is a model employed in wisdom to anticipate the calculation of individual patches from the volume of electron matches encompassing their focal titles. It’s likewise named the Gillespie-Nyholm thesis after its two abecedarian contrivers, Ronald Gillespie and Ronald Nyholm. The reason for VSEPR is that the valence electron matches encompassing a flyspeck will more frequently than not repulse one another and will, along these lines, take on a game plan that limits this repulsion. This therefore diminishes the particles energy and builds its immutability, which decides the sub-atomic computation. Gillespie has underscored that the electron-electron aversion because of the Pauli prohibition rule is more significant in deciding subatomic computation than the electrostatic shock.
The VSEPR hypothesis is utilized to anticipate the game plan of electron matches around focal particles in atoms, particularly straightforward and symmetric atoms. A focal particle is characterized in this hypothesis as an iota which is clung to at least two different iotas, while a terminal iota is attached to only another atom. For instance in the atom methyl isocyanate (H3C–N=C=O), the two carbons and one nitrogen are focal molecules, and the three hydrogens and one oxygen are terminal atoms. The math of the focal iotas and their non-holding electron matches thus decide the calculation of the bigger entire particle.
The quantity of electron matches in the valence shell of a focal not entirely settled in the wake of drawing the Lewis design of the particle, and extending it to show all holding gatherings and solitary sets of electrons. In VSEPR hypothesis, a two-fold bond or triple bond is treated as a solitary holding group.The amount of the quantity of molecules clung to a focal iota and the quantity of solitary matches shaped by its nonbonding valence electrons is known as the focal molecule’s steric number.
The electron matches (or gatherings assuming that different bonds are available) are accepted to lie on the outer layer of a circle focused on the focal molecule and will more often than not possess places that limit their common shocks by boosting the distance between them. The number of electron matches (or gatherings), subsequently, decides the general math that they will take on. For instance, when there are two electron matches encompassing the focal particle, their shared repulsion is insignificant when they lie at inverse posts of the circle. In this way, the focal iota is anticipated to take on a direct math. Assuming there are 3 electron matches encompassing the focal molecule, their shock is limited by putting them at the vertices of a symmetrical triangle fixated on the iota. Subsequently, the anticipated math is three-sided. Moreover, for 4 electron matches, the ideal course of action is tetrahedral.
Polyatomic Atoms
Polyatomic atoms are otherwise called compounds. Polyatomic molecules join with various types of powers and structure the compound. Polyatomic particles are composed of at least three molecules in a steady construction (bound state). Various particles are recognized by their sub-atomic equation, which mirrors the specific number of compositional molecules.
Polar Polyatomic Molecules
In polar polyatomic particles, the molecules of various electronegativities are available. The electronegativity contrast is high between the iotas of these polar polyatomic atoms. Instances of polyatomic components of polar sorts are nitric corrosive, sulphuric corrosive, and phosphoric corrosive.
Non-Polar Polyatomic Molecules
In nonpolar polyatomic particles, the molecules of comparative electronegativity are available. The electronegativity contrast is extremely low or zeroes in these nonpolar polyatomic particles. Instances of nonpolar polyatomic atoms are ozone, carbon dioxide, and methane.
Resonance
Resonance depicts the peculiarity of expanded adequacy that happens when the rush of an applied intermittent power (or a Fourier part of it) is original or near a characteristic rush of the frame on which it acts. While a swaying force is applied at a resounding rush of a unique frame, the frame will waver at an advanced level than when an analogous power is applied at other, non-full frequencies. Frequentness at which the response adequacy is an overall topmost is called full frequentness or reverberation frequency of the system. Small occasional powers that are close to a sonorous rush of the frame can deliver enormous acceptability movements in the frame because of the stockpiling of vibrational energy.
While every resonance structure adds to the absolute electronic construction of the flyspeck, they may not contribute also. Relegating Formal charges to iotas in the titles is one element to fete the reasonability of a reverberation structure and decide its general extent among different designs. The proper charge on a patch in a covalent beast group is the net charge the flyspeck would bear assuming that the electrons in every one of the bonds to the iota were also participating. On the other hand the proper charge on an iota in a covalent beast type is the net charge the patch would bear in the event that all bonds to the flyspeck were nonpolar covalent bonds. To decide the conventional charge on a given patch in a covalent beast kinds, use the accompanying formula:
Formal Charge = ( number of valence electrons in free orbital) − ( number of solitary brace electrons) − 1/2 ( number bond electrons)
Conclusion
The proposes of the VSEPR hypothesis are recorded beneath:
- In polyatomic particles (for example particles composed of at least three iotas), one of the constituent particles is distinguished as the focal particle to which any remaining molecules having a place with the atom are connected.
- The complete number of valence shell electron matches chooses the state of the particle.
- The electron matches tend to situate themselves in a manner that limits the electron-electron shock among them and boosts the distance between them.
- The valence shell can be considered a circle wherein the electron matches are restricted on a superficial level so that the distance between them is expanded.
- the focal particle of the atom be encircled by bond sets of electrons, then, at that point, the lopsidedly molded atom can be anticipated.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











