In organic chemistry, a hydrocarbon is an organic molecule composed entirely of hydrogen and carbon. The Hydrocarbons are the example of group 14 hydrides. Hydrocarbons are colorless, hydrophobic, and odorless. Further generalization is unattainable due to their diverse molecular structures. The combustion of fossil fuels, including their production and production processes, accounts for the vast bulk of anthropogenic hydrocarbon emissions. Plants emit hydrocarbons such as ethylene, isoprene, and monoterpenes.
An alkyne is an unsaturated hydrocarbon that contains at least one carbon—carbon triple bond in organic chemistry. The simplest acyclic alkynes have only one triple bond and no other functional groups. They are classified into a homologous series with the general chemical formula CnH2n-2. Alkynes are commonly referred to as acetylenes, while the term acetylene also refers to C2H2, which is technically referred to as ethyne in IUPAC nomenclature. Alkynes, like other hydrocarbons, are often hydrophobic.
Structure and Bonding of Alkyne
The H–C≡C bond angles in acetylene are 180°. Alkynes are rod-like due to this bond angle. Cyclic alkynes, on the other hand, are extremely rare. Benzyne is not isolable. The C≡C bond distance is significantly shorter at 121 picometers than the C=C bond distance in alkenes (134 pm) or the C–C bond distance in alkanes (153 pm).
The triple bond is extremely strong, with an 839 kJ/mol bond strength. The sigma bond contributes 369 kJ/mol of bond strength, the first pi bond contributes 268 kJ/mol, and the second pi bond contributes 202 kJ/mol. Bonding is frequently explained in terms of molecular orbital theory, which recognises the triple bond as the result of s and p orbital overlap. The carbon atoms in an alkyne bond are sp hybridized in the valence bond theory sense: they each contain two unhybridized p orbitals and two sp hybrid orbitals. Each atom’s sp orbital overlaps another to produce one sp–sp sigma bond. Each p orbital on one atom overlaps another p orbital on the other atom, generating two pi bonds in total. Each atom’s leftover sp orbital can create a sigma bond with another atom, such as hydrogen atoms in parent acetylene. The two sp orbitals are oriented perpendicular to the carbon atom.
Terminal and internal alkynes
Each acetylenic carbon in internal alkynes contains a carbon substituent. Diphenylacetylene and 3-hexyne are symmetrical examples. The formula for terminal alkynes is RC2H. Methylacetylene is one example (propyne using IUPAC nomenclature). Like acetylene, terminal alkynes are slightly acidic, with pKa values of roughly 25. They are significantly more acidic than alkenes and alkanes, which have pKa values of between 40 and 50. Terminal alkynes can have their acidic hydrogen substituted by a variety of groups, resulting in halo-, silyl-, and alkoxy alkanes. Acetylides are carbanions formed by deprotonation of terminal alkynes.
Naming alkynes
Greek prefixes are used to name alkynes in systematic chemical nomenclature. There are no extra letters added to the names. Ethyne and octyne are two examples. Triple bonds are important in parent chains with four or more carbons. It’s important to say where the triple bond is. In this case, you can write 3-octyne or oct-3-yne if the bond starts at number 3. There is only one number that can be given to the triple bond: A triple bond must be part of the parent chain when there are no superior functional groups. Even if it is not the longest possible carbon chain, it must be part of the parent chain. Ethyne is often referred to as acetylene because it has a simple name.
A triple bond is denoted by the suffix -yne in chemistry. IUPAC nomenclature is frequently used as a suffix in organic chemistry. However, substitutive nomenclature for inorganic compounds having unsaturation in the form of triple bonds can be utilized in the same way as alkynes (i.e., the name of the corresponding saturated compound is modified by replacing the “-ane” ending with “-yne”). In the case of two triple bonds, “-diyne” is used, and so on. A numerical locant preceding the “-yne” suffix, or ‘locants’ in the case of numerous triple bonds, indicates the location of unsaturation. To keep the number of participants as low as possible, the locations are carefully chosen. “-yne” can also be used as an infix to identify substituent groups that are bonded to the parent molecule in three different locations.
Occasionally, a number between hyphens is placed before it to indicate the atoms between which the triple bond exists. This suffix evolved as a shortened form of the term “acetylene.” If the final “-e” is followed by another suffix that begins with a vowel, the final “-e” is omitted.
Reactions and Application of Alkynes
Alkynes participate in a wide variety of organic reactions due to their reactive functional group. This application was pioneered by Ralph Raphael, who published the first book outlining their versatility as synthesis intermediates in 1955.
Hydrogenation
Alkynes, being more unsaturated than alkenes, undergo reactions that demonstrate their “doubly unsaturated” state. Alkynes can add two equivalents of H2, whereas an alkene can only add one. Alkynes add one or two hydrogen equivalents depending on the catalysts and circumstances. Partial hydrogenation, in which only one equivalent is added to produce the alkene, is usually preferable, as alkanes are less useful:
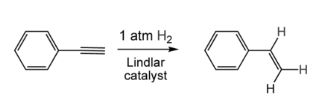
The largest industrial application of this technology is the conversion of acetylene to ethylene in refineries (steam cracking alkanes produce a small amount of acetylene, which is selectively hydrogenated in the presence of a palladium/silver catalyst). For more complex alkynes, such as phenylacetylene to styrene, the Lindlar catalyst is frequently recommended to avoid the production of the alkane.
Similarly, alkynes are halogenated to form alkene dihalides or alkyl tetrahalides:
RC≡CR′ + H2 → cis-RCH=CR′H
RCH=CR′H + H2 → RCH2CR′H2
By adding one equivalent of H2 to internal alkynes, cis-alkenes are formed.
Addition of halogens and related reagents
It’s common for alkynes to have two equivalents of halogens and hydrogen halides that they can add.
RC≡CR′ + 2 Br2 → RCBr2CR′Br2
For silanes, boranes, and similar hydrides, the insertion of nonpolar E–H bonds across C≡C is universal. When alkynes are hydroborated, vinylic boranes are formed, which oxidise to the appropriate aldehyde or ketone. The thiol-yne reaction requires a thiol as the substrate.
Hydrogen halide addition has long been of interest. Acetylene and hydrogen chloride react in the presence of mercuric chloride to form vinyl chloride. While this process has been abandoned in the West, it continues to be the primary way of production in China.
Hydration
Acetaldehyde is formed when acetylene is hydrated. The process begins with the synthesis of vinyl alcohol, which tautomerizes to produce the aldehyde. This reaction was originally a significant industrial process, but has since been supplanted by the Wacker reaction. This process happens naturally, with acetylene hydratase as the catalyst.
Hydration of phenylacetylene produces acetophenone, and the hydration of 1,8-nonadiyne to 2,8-nonanedione is catalysed by (Ph3P) AuCH3
PhC≡CH + H2O → PhCOCH3
HC≡C(CH2)5C≡CH + 2H2O → CH3CO(CH2)5COCH3
Uses of Alkyne
- Because ethyne burns extremely hot, it is frequently used in oxyacetylene gas welding and cutting. When ethyne is burned with oxygen, the resulting fire reaches a temperature of around 3600 Kelvin.
- Acetylene’s successor alkyne is used as a fuel, with millions of kg produced annually via partial oxidation of gaseous petrol. A part of these alkynes is used to create mixes such as ethanoic corrosive, acrylic corrosive, and ethanol.
- The most common usage of ethyne is to create natural combinations such as ethanol, ethanoic corrosive, and acrylic corrosive. Additionally, it is used to manufacture polymers and raw ingredients for them.
- Acetylene is composed of two components: carbon and hydrogen. This reaction generates a tremendous amount of heat, which can cause the gas to glow independent of the presence of air or oxygen.
- Alkynes are frequently used as starting materials in the synthesis of a large number of natural mixes with mechanical relevance, for example, chloroprene, vinyl chloride, and so on.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






