Diazonium compounds or salts come in the category of organic compounds having triple bonds between nitrogen atoms. They have either an aryl (benzene ring) or alkyl on the other side. Diazonium salts are also known as the azo dyes’ intermediate phase. The double nitrogen (diazo) is present in ionic salts. It is where chloride molecules can replace nitrogen atoms.
Preparation Of Diazonium Salt
The overall process of making diazonium salts is simple.
The following are the steps:
- The Diazotization or dissociation process is leveraged to make an organic compound. The process converts primary aromatic amines into diazonium salts.
- The Diazonium group is known as highly unstable even in normal conditions. Hence, it is not stored normally and used instantly after preparation.
- Nitrous acid and aromatic amines are commonly used to synthesise diazonium salts. Nitrous acid and aniline react to produce benzene diazonium chloride as a result of the reaction.
- Because nitrous acid is a highly toxic gas, it is usually made in situ by reacting NaNO2 with a mineral acid.
- Temperature is another factor that affects product formation during the preparation of diazonium salts; most salts are stable below 5º
- Keeping the reaction temperature below 5ºC prevents the diazonium group from decomposing into N2.
Chemical Reactions Of Diazonium Salt
Diazonium salt reactions are essential because we can use them to make the building blocks for a wide range of organic reactions. Sandmeyer reactions and other mechanisms are two types of these reactions.

Sandmeyer Reaction
Aryl diazonium salts can be further transformed into aryl halides through the Sandmeyer reaction, also known as the radical-nucleophilic aromatic substitution. Sandmeyer’s reaction enables Benzene to undergo numerous transformations, including halogenation and Benzene. This method enables the substitution of an aromatic amine in copper(I) salts by a nucleophile, which can be cyanide, thiols, or halide anions.

You can start by taking diazonium salts and introducing them to copper compounds, such as copper (I) chloride. The Sandmeyer reaction transforms copper (I) chloride into aryl chloride. Swiss chemist Traugott Sandmeyer discovered it in 1884. It is also recognized for the immediate loss of nitrogen in the diazonium salt. The process is triggered by Chloride atoms, present via the copper (I) chloride reagent.
The Reaction Involves The Following Processes
- For every aryl amine, the general precursor is its nitro compound. It deactivates the benzene ring and electrophilic replacements happen across meta locations.
- Electrophilic substitution occurs at ortho and para locations when the amine’s nitro group reduces, activating the aromatic ring.
- Here, we convert the aryl amine to diazonium ions, which we can use in subsequent reactions.
Other Reactions
Schiemann Reaction
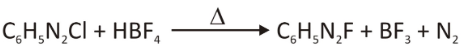
When we substitute the (X–) ion of the diazonium salt with the tetrafluoroborate (BF4–) ion when treated with HB4, it forms aryl fluorides. Stable diazonium tetrafluoroborate salts contain fluorine. When we heat, it can act as a nucleophile and lose its nitrogen, resulting in aryl fluoride and the byproducts BF3 and N2. The reaction is the Schiemann reaction.
Phenol Synthesis
When we heat the aryl diazonium salt with acid and water, we add hydroxyl groups (OH). These phenols are valuable because we use them in pharmaceuticals and drug research.
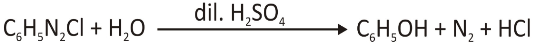
Aryl Iodide
The aryl diazonium salts also react with potassium iodide to produce aryl iodide.
Reduction of the Diazonium Salt from the Amino Group
By reacting with hypophosphorous acid (H3PO2), the aryl diazonium salts can remove the nitro (amino) group and form C-H.
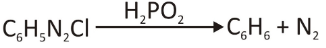
Diazonium Coupling Reactions
The electron-rich nucleophiles can only couple with the terminal nitrogen rather than the inner nitrogen due to the positive charge displacement on the nitrogen atoms, resulting in azo dyes.
C6H5N2Cl + C6H5OH → C6H5 –N2–C6H4 –OH
We use diazo coupling reactions in many fields, such as producing synthetic dyes for colours like yellow, red, orange, etc. Because of their colour-related abilities, We call them azo dyes, which are present in cis and transformations.
Formation Of Diazonium Ions
The first key reagent is sodium nitrite (NaNO2) or nitrous acid (HNO2). Sodium nitrite is an easily handled salt, whereas HNO2 is a volatile liquid. When using NaNO2, HCl converts it into HNO2 with the help of acid like HCl.
The primary purpose of HCl is to convert HNO2 into the powerful electrophile NO+, also known as the “nitrosonium ion,” which is the key electrophile in the diazonium salt reaction. Protonation forms the nitrosonium ion by the OH and results in water loss.
The amine and the nitrosonium ion reaction also requires acid from the diazonium ion.
Diazo Coupling
Dye derivatives derived from azobenzene, the essential structure of which consists of a nitrogen-nitrogen double bond, are present in surprising numbers in our daily lives. Red, orange, and yellow are typical azo dye colours.
A diazonium salt reacts with an aromatic compound rich in electrons to produce azo dyes. Only electron-rich aromatic species can attack diazonium salts.
Conclusion
Diazonium salts come in the organic group. It shares the same functional group R – N2+ X, where R denotes some organic residue (such as an alkyl or aryl group) and the X is an organic or inorganic anion (a halogen, for example). Diazonium salts having R as an aryl group, serves as essential intermediates in the organic synthesis of azo dyes. We add the suffix diazonium to the parent hydrocarbon, followed by the anion’s name, such as chloride or hydrogen sulphate. The ion N2+ is a diazonium group—benzene-diazonium chloride (C6H5N2+Cl–). Diazonium salts are colourless crystalline solids that dissolve quickly in water. In a dry environment, they decompose quickly.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




