A Ziegler–Natta catalyst is a catalyst used in the synthesis of 1-alkene polymers. It is named after Karl Ziegler and Giulio Natta (alpha-olefins). There are two types of Ziegler–Natta catalysts used, each with its own solubility:
In polymerization operations, heterogeneous supported catalysts based on titanium compounds are utilized in conjunction with cocatalysts, organoaluminum compounds such as triethylaluminum, AlC2H53. This type of catalyst is the most common in the business. [1]
Homogeneous catalysts are commonly composed of titanium, zirconium, or hafnium compounds. They’re generally combined with methylaluminoxane, a distinct organoaluminum cocatalyst (or methylalumoxane, MAO). Metallocenes are commonly used in these catalysts, but they also include multidentate oxygen and nitrogen-based ligands. [2]
Terminal alkenes (ethylene and alkenes with the vinyl double bond) are polymerized using Ziegler–Natta catalysts:
n CH2=CHR → -[CH2-CHR]n-
HISTORY OF THE CATALYST:
German Karl Ziegler and Italian Giulio Natta shared the Nobel Prize in Chemistry in 1963 for discovering the first titanium-based catalysts and using them to make stereoregular polymers from propylene. Since 1956, Ziegler–Natta catalysts have been utilized in the commercial production of various polyolefins. In 2010, more than 100 million tonnes of polymers, elastomers, and rubbers were generated from alkenes using these and related catalysts (particularly Phillips). These polymers make up the world’s largest-volume commodity plastics as well as the world’s largest-volume commodity chemicals.
Phillips Petroleum employees discovered that chromium catalysts are particularly effective for low-temperature ethylene polymerization in the early 1950s, launching important industrial breakthroughs that culminated in the Phillips catalyst. After a few years, A few years later, Ziegler discovered that combining titanium tetrachloride (TiCl4) with diethyl aluminum chloride (AlC2H52Cl) produced polyethylene with equal activity. Natta created the first isotactic polypropylene by combining crystalline -TiCl3 with AlC2H53. [3] Ziegler catalysts are titanium-based systems for ethylene conversions, whereas Ziegler–Natta catalysts are titanium-based systems for propylene conversions. Magnesium chloride was discovered in the 1970s to considerably improve the activity of titanium-based catalysts. These catalysts were so active that the remaining titanium in the product was no longer eliminated. They permitted the creation of noncrystalline copolymers and the commercialization of linear low-density polyethylene (LLDPE) resins. [4]
BASF also developed a gas-phase, mechanically stirred polymerization process for polypropylene in the 1960s. During that time, Union Carbide launched the Unipol method, the first gas-phase fluidized-bed polymerization process, in 1968 to make polyethylene. The Unipol technique was
developed to make polypropylene in the mid-1980s.The fluidized-bed process’ characteristics, such as its simplicity and product quality, have made it widely accepted around the world. The fluidized-bed method is one of the two most extensively used polypropylene production systems today. [5]Ziegler–Natta catalysts with magnesium chloride support were first introduced in the 1970s. These catalysts have improved activity, allowing for the elimination of time-consuming steps in the process. Deashing (removal of residual catalyst) and removal of undesired amorphous polymer were two of the steps that were skipped. [6]
PREPARATION OF CATALYST:
Today’s Ziegler-Natta catalysts typically comprise a variety of transition metal halides, such as titanium, vanadium, chromium, and zirconium, as well as organic non-transition metal derivatives, particularly alkyl aluminum compounds.
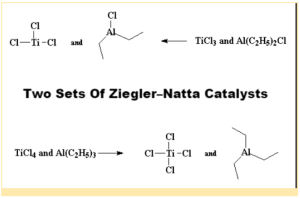
Transition metal halides belonging to groups IV-VIII are commonly interacted with organometallic compounds belonging to groups I – III in the contemporary periodic table to prepare or acquire Ziegler-Natta catalysts. A blend of titanium tetrachloride (TiCl4) and trimethylaluminum (AlC2H53) is a popular example. The catalysts have shown to be really beneficial.
MECHANISM OF ZIEGLER NATTA CATALYST:
In coordination polymerisations, the Ziegler-Natta catalyst is utilized, and it involves complexes generated between a transition metal and the electrons of a monomer. The insertion of monomers at the end of the expanding chain, where the transition metal ions are attached, is typically how polymerisation is performed. The entering monomers are all coordinated at the same moment at unoccupied orbital locations, resulting in lengthy polymer chains. The C=C bond is also included into the TiC bond at the active center.
Finally, the chain-growth polymerisation reaches the termination stage, which produces “dead” polymers (the intended result). Anionic polymerisation, which creates linear and stereo-regular polymers, is comparable to these processes.
CLASSIFICATION OF ZIEGLER NATTA CATALYST:
Heterogeneous
These are titanium compounds-based catalysts. They are coupled with organoaluminum compounds and cocatalysts during polymerisation operations.
Homogeneous
These are reliant on complexes of Hf, Ti, or Zr. Metallocenes are common, although they also comprise ligands based on nitrogen and multidentate oxygen.
APPLICATION OF THIS CATALYST:
The Ziegler-Natta catalyst polymerization reaction is a versatile and valuable polymerization method. Some of the most common applications for this catalyst include:
1.High-density and low-density polyethylene are made with them.
2.Carbon nanotube nanocomposites, thermoplastic polyolefins, polybutylene, crystalline polypropylene, and thermoplastic polyolefins are all made.
LIMITATIONS:
The Ziegler-Natta polymerization process has a few drawbacks, such as the fact that it does not operate with all monomers. Polymerization using Ziegler-Natta cannot create compounds like poly (vinyl chloride). Acrylates are another example.
CONCLUSION:
A Ziegler–Natta catalyst is a catalyst used in the synthesis of 1-alkene polymers. It is named after Karl Ziegler and Giulio Natta (alpha-olefins). There are two types of Ziegler–Natta catalysts used, each with its own solubility. In this chapter we have discussed about Ziegler natta catalyst , discovery, preparation, mechanism and applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




