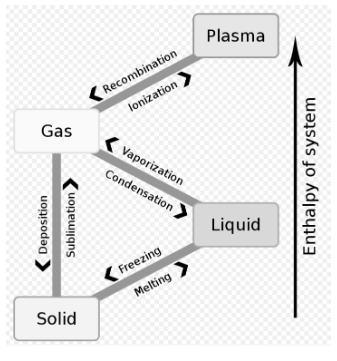
We notice several changes occurring in our daily lives all around us. When we leave a glass of cold water unattended for an extended period of time, the surface of the glass becomes completely coated in liquid droplets. When milk is kept over a gas flame, bubbles begin to develop when the temperature reaches a certain point. When heated over a flame, a metal ring increases in diameter, water solidifies in the freezer, and ice cream melts at normal temperature. These are referred to as Phase Changes (also known as Phase Transitions). The distinct phases of substances are defined by a spectrum of thermodynamic factors, with these stable phases being the most stable. Additional phases include amorphous, colloidal, crystalline, plasma, and glassy. The phase associated with the Plasma state is thought to be the most widespread.
The thermodynamic phase of plasma occurs when the number of charged particles contained within the matter approaches unity, resulting in the ionisation of the matter’s gas, as both positively and negatively charged particles become equal in number. As a result, plasma is frequently referred to as the fourth phase of matter, following the solid, liquid, and gaseous phases.
What is Phase Transition?
During phase transitions (or phase changes) in chemistry, thermodynamics, and a lot of other fields, things move from one state to another with different values of certain parameters. A lot of people use the term “change of state” to refer to changes in the three basic states of matter: solid, liquid, and gas, as well as plasma in a few rare cases.
It’s like how the physical properties of a phase of a thermodynamic system and states of matter are the same. Phase transition: When a certain medium changes, some of its properties, such as temperature, pressure or other things, can change quickly. This happens because of changes in the outside world, such as these things. As an example, when a liquid heats up to the boiling point, it may turn into a gas, causing a big change in its volume right away. The measurement of the external conditions at which the transformation takes place is called the phase change. Phase changes happen all the time in nature and are used in many modern technologies.
First order phase transition
First-order phase transitions are those in which there is a latent heat transfer. An energy transition occurs when a system either absorbs or releases a fixed (and often significant) amount of energy per unit volume of the system in question. It is during this process that the temperature of the system will remain constant even when more heat is introduced: the system is in a “mixed-phase regime,” in which certain sections of it are in a state of transition while other parts are not. The melting of ice or the boiling of water are both well-known examples of this (the water does not instantly turn into vapor, but forms a turbulent mixture of liquid water and vapor bubbles). It has been demonstrated by Yoseph Imry and Michael Wortis that quenched disorder can widen a first-order transition. In other words, the transformation is completed across a limited range of temperatures, yet phenomena such as supercooling and superheating continue to exist, and hysteresis is detected during the thermal cycling process.
Second order phase transition
Second-order phase transitions, also known as “continuous phase transitions,” are called that because they happen over time. Their susceptibility is different, the length of their connections is infinity, and their correlations fall off in a power law way when they reach criticality. For example, the ferromagnetic transition, superconducting transition, and superfluid transition are all second-order phase transitions. For a Type-I superconductor, the phase transition is second-order at zero external field, and for a Type-III, the phase transition is second-order for both normal-state–mixed-state transitions and mixed-state–superconducting-state transitions, as well. In contrast to viscosity, the thermal expansion and heat capacity of amorphous materials change quickly at the glass transition temperature, which makes it easier to see with differential scanning calorimetry. It was Lev Landau who came up with a theory about second-order phase transitions.
It isn’t just simple phase transitions that happen on their own. There are transition lines and multicritical points that happen when you change things like the magnetic field or composition.
A lot of transitions are called infinite-order phase transitions because they happen over and over. They are connected but don’t break any rules. Most people know about the Kosterlitz–Thouless transition in the two-dimensional XY model, but there are many more. There are many quantum phase transitions that fall into this category, such as in two-dimensional electron gas.
People who make polymers and other liquids that can be supercooled far below the melting point of the crystalline phase see this transition happen. This isn’t typical in a lot of ways. In this case, it is not a change in the thermodynamic ground state. It is widely thought that the true ground state is always crystallised. Because of how it has been heated, glass can have different densities, entropies, and other properties that are different than they would be if it had not been heated at all. So, the glass transition is mostly a process: as a liquid cools, more and more of its internal degrees of freedom fall out of equilibrium. Some theoretical methods say that in the case of infinitely long relaxation times, there will be an underlying phase transition.
Conclusion
Because the three fundamental phases of matter are Solid, Liquid, and Gas, as previously stated, it is critical to address the possibility of phase shift. According to its definition, phase transition occurs when a substance undergoes a transformation and takes on a new form. For example, when we freeze water in the freezer, it transitions from the liquid to the solid phase, resulting in a phase change in the water.
Solid: The energy of the atoms decreases in this state of matter, resulting in the development of a three-dimensional structure. Consider an ice cube.
Liquid: In this state of matter, the matter’s structure is pliable, taking on the shape of the vessel into which it is poured. For instance, a jar of water.
In this state of matter, the matter’s structure is gaseous, which means it cannot take on any shape or structure. For instance, the mist produced by boiling water.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




