Alkanes are open-chain saturated hydrocarbons, Because of their inertness, they are called paraffins (Latin: param = little, affinis =affinity) because they exhibit little affinity for most reagents such as acids, bases, oxidising and reducing agents at common temperatures and pressures. Hybridisation of each Carbon-atom in an alkane molecule is sp3. Each sp3 -hybridised carbon atom produces four C-bonds that point towards the tetrahedron’s corners. As a result, an alkane’s carbon atoms have a tetrahedral structure. C n H 2n+2 [where n:1, 2,…] is the chemical formula for alkanes. Their general formula is RH (R: alkyl group).
Chemical Properties of Alkane
Oxidation of Alkane
Combustion Reaction
Alkanes burn in the presence of excess oxygen to manufacture carbon dioxide and water without releasing a significant amount of heat. Alkanes are utilised as fuels because of this. The main components of LPG used for household cooking are n-butane, propane, and isobutane, as well as a minor proportion of ethyl mercaptan.
Controlled Oxidation
This reaction produces alcohols, aldehydes, and carboxylic acids when alkanes are exposed to oxygen at high temperatures and pressures in the presence of a metal or metallic oxide catalyst.
Substitution Reaction
Substitution reactions are the most common reaction in saturated hydrocarbons. Any monovalent atom or group replaces the hydrogen atom linked to the carbon atom in these processes.
Halogenation
In the reaction of halogens with alkanes in presence of light, heat (250 – 400 degrees C) or catalyst, the hydrogen atoms of the alkanes are easily replaced by halogen atoms to give haloalkanes and hydrogen halide. This is called a halogenation reaction. A chemical reaction between an alkane and a halogen in which one or more hydrogen atoms are replaced by halogens are known as halogenation reactions.
Two common halogenation processes are chlorination and bromination. Iodination is too slow and fluorination is too fast. In the presence of light or when heated, methane interacts with chlorine as follows.
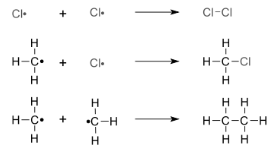
Mechanism
The free radical chain mechanism is used to carry out the reaction. The three steps of this mechanism are start, propagation, and termination.
- i) chain initiation: UV radiation initiates the chain, which results in the homolytic fission of chlorine molecules into free radicals (chlorine atoms).
Because C-C and C-H bonds are stronger than Cl-Cl bonds, we chose Cl-Cl for fission.
ii) PROPAGATION: This is how it works:
a) A chlorine free radical attacks a methane molecule and breaks the C-H link, producing a methyl free radical.
b) The formed methyl free radical reacts with the second molecule of chlorine to produce chloromethane (CH3Cl) and a chlorine free radical, as shown below.
c) The chlorine free radical then returns to step (a), and both steps (a) and (b) are repeated numerous times, forming a chain reaction.
iii) Chain termination: Reactions can sometimes come to a halt due to reactant consumption, and the chain is terminated by a combination of free radicals.
Nitration
Nitration is the reaction in which the hydrogen atom of an organic compound is replaced with a nitro (- NO2) group. Nitration occurs when an alkane and fuming HNO3 vapour are heated at higher temperatures (400-475 degree C) under pressure to produce nitroalkane.
During the nitration reaction, any type of hydrogen atom in an alkane molecule can be replaced by the – NO2 group. C-C bond cleavage may also occur, resulting in the creation of a mixture of lower nitroalkanes.
Aromatisation
At high temperatures and in the presence of a catalyst, alkanes with six to ten carbon atoms are transformed into homologous benzene. Aromatization is the term for this process. It occurs when alkanes undergo simultaneous cyclisation and dehydrogenation.
Benzene is produced by passing n-hexane over Cr2O3 supported on alumina at 873 K. When an alkene is passed over a suitable catalyst heated at 500-750 degree C, one molecule of hydrogen is eliminated from a molecule of the alkane to liberate an alkene.
Reaction with Steam
Methane decomposes into carbon monoxide and hydrogen gas when it combines with steam at 1273K in the presence of nickel. Steam reforming is a well-established industrial technique for producing H2 gas from hydrocarbons, and it is used to produce H2 gas from methane.
Pyrolysis is the Breakdown of a Substance
Pyrolysis is described as the application of heat to break down an organic substance into smaller fragments in the absence of oxygen. Thermal decomposition of organic compounds is called pyrolysis. Pyro denotes “fire,” and lysis denotes ” separate.” Alkane pyrolysis is known as cracking. When alkane vapours are pushed through red-hot metal in the absence of air, they decompose into simpler hydrocarbons. The type of the alkane, temperature, pressure, and the presence or absence of a catalyst all influence the final product. Alkanes become more difficult to fracture as their molecular weight and branching grow. In the petroleum sector, cracking is crucial.
Isomerisation
Isomerisation is the chemical transformation of a molecule into any of its isomeric forms. In the presence of AlCl3 and HCl at 298 k, normal alkanes can be transformed to branched alkanes. Conversion of one isomer of a compound into another isomer is called isomerism, when an n-alkane is heated at high temperature (300 degree C) in the presence of a catalyst (anhydrous AlCl3 /HCl or AlBr3 /HBr) it gets converted to a branched chain isomer. This is an extremely important industrial process. Isomerizing the components of gasoline improves its quality.
Conclusion
Alkanes, commonly known as hydrocarbons, are chemical compounds that include carbon (C) and hydrogen (H) atoms. Alkanes have only one bond in their chemical structure. Because these alkanes only have single bonds, each carbon atom is saturated with hydrogen atoms, they are referred to as saturated. As a result, alkanes are known as saturated hydrocarbons.
Alkanes are important chemical raw materials as well as the main component of gasoline and lubricating oils. Natural gas is mostly composed of methane and ethane which is used for heating, cooking, and power generation (gas turbines) and for many other purposes. Natural gas can be liquefied for transportation reasons by applying pressure and cooling it (LNG = liquid natural gas).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






