The term “oxidation” was first applied to reactions in which metals react with oxygen in the air to form metal oxides.
When iron is exposed to air in the presence of water, for example, the iron oxidises and becomes rust. Aluminium metal produces a continuous, transparent layer of aluminium oxide on its surface when exposed to air.
In both circumstances, the metal gains a positive charge by donating electrons to an oxygen molecule’s neutral oxygen atoms. The oxygen atoms gain a negative charge as a result, forming oxide ions.
The term “reduction” originally referred to the mass loss that occurred when a metal oxide was heated with carbon monoxide, a common method for extracting metals from their ores.
Oxidation States Assigning
In binary ionic compounds, assigning oxidation states to the elements is simple: the oxidation states of the elements are identical to the charges on the monatomic ions.
You learnt how to estimate the formulas of simple ionic compounds based on the sign and amount of the charge on monatomic ions generated by neutral elements in the previous section. Sodium chloride, magnesium oxide (MgO), and calcium chloride are examples of such chemicals (CaCl2).
Atoms in covalent compounds, on the other hand, share electrons. However, by considering the elements as if they were ionic, we can still assign oxidation states to them (that is, as if all the bonding electrons were transferred to the more attractive element).
Although oxidation states in covalent compounds are rather arbitrary, they are important accounting tools for understanding and predicting many reactions.
Oxidation State Assignment Rules
In any pure element, whether monatomic, diatomic, or polyatomic, the oxidation state of an atom is zero.
A monatomic ion’s oxidation state is the same as its charge—for example, Na+ = +1, Cl_ = 1.
Fluorine’s oxidation state in chemical compounds is always 1. Except when coupled with oxygen or other halogens, other halogens normally have oxidation states of 1.
In nonmetal compounds, hydrogen has an oxidation state of +1, while in metal compounds, it has an oxidation state of 1.
The oxidation state of oxygen is typically given to 2 in compounds, with two exceptions: in compounds containing oxygen–fluorine or oxygen–oxygen bonds, the oxidation state of oxygen is decided by the oxidation states of the other elements present.
The charge on a neutral molecule or ion must be equal to the sum of the oxidation states of all the atoms in the molecule or ion.
Numbers of Oxidation
Ions have an electrical charge, which is either negative or positive depending on whether they have received or lost electrons.
The existence of ions implies that electrons are transferred from one atom to another, resulting in positive and negative charges.
The assignment of imaginary charges called oxidation numbers to atoms with polar covalent connections is a helpful application of this concept. The overall objective is to give the more electronegative element the shared electrons in each bond.
Use the water molecule as an example in a standard Lewis diagram, as shown in Figure.
Figure of H2O Lewis structure.
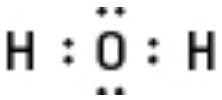
Oxidation-Reduction Reactions
Redox reactions are composed of two parts, a reduced half and an oxidised half, that always occur together. The reduced half gains electrons and the oxidation number decreases, while the oxidised half loses electrons and the oxidation number increases.
Simple ways to remember this include the mnemonic devices OIL RIG, meaning “oxidation is loss” and “reduction is gain.” There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken up by another species in the reduction half reaction.
The two species that exchange electrons in a redox reaction are given special names:
The ion or molecule that accepts electrons is called the oxidising agent – by accepting electrons it oxidises other species.
The ion or molecule that donates electrons is called the reducing agent – by giving electrons it reduces the other species.
Hence, what is oxidised is the reducing agent and what is reduced is the oxidising agent.
A good example of a redox reaction is the thermite reaction, in which iron atoms in ferric oxide lose (or give up) O atoms to Al atoms, producing Al2O3 .
Conclusion
The term “oxidation” was first applied to reactions in which metals react with oxygen in the air to form metal oxides.
When iron is exposed to air in the presence of water, for example, the iron oxidises and becomes rust. Aluminium metal produces a continuous, transparent layer of aluminium oxide on its surface when exposed to air.
The term “reduction” originally referred to the mass loss that occurred when a metal oxide was heated with carbon monoxide, a common method for extracting metals from their ores.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






