One of the most prevalent chemical molecules we encounter in our daily lives is hydrogen peroxide. It can be found in hair bleaching products as well as medical products. In this session, we will dive deeper into the topic of hydrogen peroxide, covering its structure, properties, and more.
Hydrogen peroxide is a chemical compound composed of hydrogen and water molecules. H2O2 is the chemical formula for it. When hydrogen peroxide is pure, it appears as a clear liquid with a faint pale blue coloration. It is more viscous than water. It is, however, a thermodynamically unstable liquid that decomposes when exposed to light. This chemical can be present in the human body as well.
Structure of Hydrogen peroxide
Peroxide is a chemical compound that contains the peroxide ion (O2²–). The peroxide ion is made up of a single bond between two oxygen atoms and is denoted by the symbol (O–O)²–. It is an extremely potent oxidant.
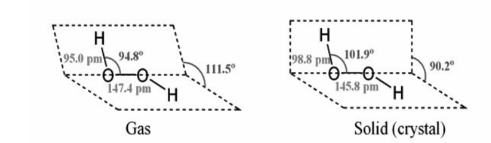
The peroxide link connects two oxygen atoms in a non-planar molecule called hydrogen peroxide.
It’s written in a book-like format. A single connection connects each oxygen atom to each hydrogen atom. The two bonds do not lie in the same plane because lone pairs of electrons repel each other across oxygen atoms. The dihedral (interplanar) angle between the two planes of hydrogen peroxide is 111.5° while it is gaseous, but 90.2° when it is crystalline. This is caused by intramolecular hydrogen bonding.
The valence bond theory can also be used to describe the structure of H2O2, as both oxygen atoms are sp-hybridized. Lone pairs of electrons occupy two of these hybrid orbitals on each oxygen. The third hybrid orbital makes an O-H sigma bond with the s-orbital of a hydrogen atom, while the fourth forms a sigma bond with the second oxygen atom’s half-filled hybrid orbital.
In both the gaseous and crystalline phases, the hydrogen bond parameters are:
Preparation of Hydrogen peroxide
Laboratory preparation
From sodium peroxide (Merck’s Process): In this technique, sodium peroxide is added in minute amounts to a weak sulphuric acid (20%) solution covered by ice and regularly stirred. Crystals of Na2SO4. 10H2O occurs as the solution is cooled further, and they can be filtered out. A 30 percent hydrogen peroxide aqueous solution is used.
From barium peroxide: A 20 percent ice-cold sulphuric acid solution is used to treat a hydrated barium peroxide (BaO2.8H2O) paste prepared in ice-cold water. The BaSO4 precipitate, which is white in appearance, is removed using filtration. Only around 5% of the H2O2 is left in the solution.
Because barium sulphate forms a protective coating around H2O2 that prevents it from continuing the chemical reaction, this approach is ineffective. Hydrogen peroxide is progressively decomposed by the Ba2+ ions in the solution. As a result, the solution is not suitable for long-term storage. Instead of sulphuric acid, phosphoric acid is used to test this. In the absence of Ba2+ ions, the barium phosphate that develops is completely precipitated, and there is no risk of hydrogen peroxide breakdown.
Industrial preparation
A sulfuric acid solution is electrolyzed to produce: A 50 percent sulphuric acid solution is electrolyzed in a cell. The anode produces peroxodisulfuric acid, which causes hydrogen to be released at the cathode.
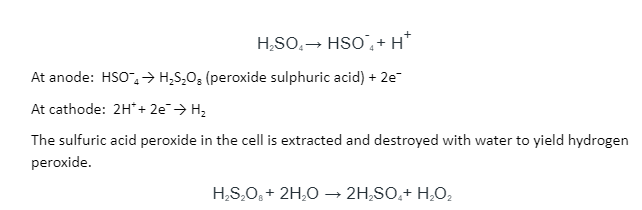
Although hydrogen peroxide distils, sulphuric acid with a high boiling point does not. The production of hydrogen peroxide can be boosted by electrolyzing a mixture of ammonium sulphate and sulphuric acid in equal amounts.
The ammonium peroxide sulphate produced at the anode is distilled with water to form hydrogen peroxide.
Uses of Hydrogen peroxide
- Hydrogen Peroxide has been utilised for wound disinfection since the dawn of time, owing to its inexpensive cost and quick availability compared to other antiseptics. However, because it kills freshly created skin cells, it is now suspected to hinder healing and cause scarring.
- Dermal exposure to dilute Hydrogen Peroxide solutions can produce skin bleaching or whitening.
- Hydrogen peroxide is also used extensively in the cosmetic industry.
- Most whitening toothpastes contain it. To produce homemade toothpaste, combine this chemical with baking soda and salt.
- This substance is commonly used in the manufacture of complementary and alternative medicine. Although there is no proper evidence of success, and in some situations, it may even be life-threatening, the usage of hydrogen peroxide can heal a variety of ailments, including emphysema, influenza, AIDS, and cancer.
- In the rocket industry, hydrogen peroxide can be employed as a propellant. In a jet back, rocket-belt Hydrogen Peroxide propulsion systems are used.
- As a result, Hydrogen Peroxide is one of the most significant molecules in fish aeration.
- Horticulture: Some horticulturists and hydroponics users recommend watering solutions with a weak Hydrogen Peroxide solution.
- Organic compound production: Hydrogen Peroxide is used to make a variety of organic Peroxides, with benzoyl Peroxide being one of the most common. It’s utilised in polymerizations, as well as as a flour bleaching agent and an acne treatment.
- Hydrogen peroxide has also been used to eliminate organic pollutants in some waste-water treatment systems.
- Hydrogen peroxide is used to sterilise a variety of surfaces, including surgical instruments, and it can also be used to sterilise rooms as a vapour (VHP).
- Because it degrades to water and oxygen, hydrogen peroxide is a safer alternative to chlorine-based bleaches for the environment. It is also generally acknowledged as safe as an antibacterial agent by the US Food and Drug Administration (FDA).
- In the textile and paper industries, hydrogen peroxide is utilised as a bleaching agent. According to data, about 60% of the world’s production of Hydrogen Peroxide is utilised for pulp and paper bleaching.
- In everyday life, hydrogen peroxide can be used as a hair bleach as well as a light disinfectant.
- One of the most important industrial applications of this molecule is the production of sodium percarbonate and sodium perborate, which are mild bleaches used in laundry detergents. Sodium percarbonate is a chemical that is found in products like
OxiClean and Tide laundry detergent. When dissolved in water, it produces hydrogen peroxide and sodium carbonate.
CONCLUSION
H2O2, a colourless liquid often generated as aqueous solutions of varying strengths, is primarily used for bleaching cotton and other textiles and wood pulp, in the synthesis of other chemicals, as a rocket propellant, and for cosmetic and therapeutic applications. Solutions containing more than 8% hydrogen peroxide are corrosive to the skin.
Hydrogen peroxide, which was discovered as a chemical compound in 1818, is the most basic member of the peroxide class. The primary manufacturing procedures include interactions of oxygen from the air with certain chemical molecules, particularly anthraquinone or isopropyl alcohol.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






