Calcium carbonate has the chemical formula CaCO3 which is an inorganic substance. The majority of it is located in the earth’s crust. It comes in a variety of forms, including limestone, marble, and calcite. Calcium carbonate can be found in natural forms such as aragonite, vaterite, and calcite, as well as in industrial forms such as limestone, marble, chalk, and travertine. Eggshells, snail shells, seashells, and pearls all include it as a biological component. It’s also a component of dark green vegetables, which have a high calcium carbonate content. It can be used to make calcium supplements and antacids in medicine.
Calcium carbonate’s properties:
- Calcium carbonate is a powdery substance.
- It decomposes in the presence of acids, releasing carbon dioxide.
- It produces calcium oxide as a byproduct of heat breakdown.Quick lime is another name for calcium oxide. Calcination is the process of breakdown that produces calcium oxide.
- It produces calcium bicarbonate when it combines with water.
- It exhibits characteristics that are similar to carbonates.
- Erosion is caused by the interaction of calcium carbonate with water.
Hard water is formed when calcium carbonate reacts with water.
- Ikaite is the hexahydrate form of calcium carbonate that is stable at temperatures above 80°C.
Extraction:
Calcium carbonate is typically obtained by mining and processing natural mineral sources. It can also be manufactured chemically by mixing quicklime (calcium oxide, CaO) with water to get calcium hydroxide (Ca(OH)2), which is then treated with carbon dioxide to generate calcium carbonate salt.

Calcium carbonate is widely made in a variety of ways:
Method 1: The most common method for obtaining a substantial volume of calcium carbonate is mining or quarrying. Quarrying from pure sources extracts the pure form of calcium carbonate.
Calcium carbonate is made using the second method, which uses calcium oxide.Calcium hydroxide is formed when calcium oxide is combined with water. Calcium carbonate is generated once the product is treated with carbon dioxide. The solution is precipitated to obtain it.
Method 3: Calcium carbonate can also be made by combining slaked lime with carbon dioxide.Calcite is a type of calcium carbonate that is produced by passing carbon dioxide through slaked lime.
Method 4: Another method is to obtain calcium carbonate as calcite. Calcium chloride is utilised in this procedure. Calcium carbonate in the form of calcite is made by mixing calcium chloride with sodium carbonate.
Calcium carbonate has a variety of applications:
1) Calcite Powder in Paper: Micronized calcite has been used in the paper industry to make paper and cardboard by mixing it with cellulose in various amounts. Calcite naturally improves the whiteness of paper.
2) Use of Calcite Powder in Paint: Our carbonates’ outstanding whiteness prevents interference with the paint colours and contributes to their opacity, allowing the paint to cover the surfaces without dripping.
3) Used in plastic: Calcite Powder is commonly used as a mineral filler in plastics to achieve excellent humidity retention and improve the consistency and dryness of the final mass. These polymers are used in a variety of products, including bags, pipelines, automobile parts, furniture, and containers.
4) Calcite in Cosmetics: Calcite is commonly found in cosmetics such as make-up, soaps, and toothpastes. Calcite has a lot of advantages when it comes to cosmetics.
5) Calcite in Glass: Calcite powder for the glass industry must contain a high and consistent percentage of CaO and be free of color-inducing impurities such as Fe2O3 and other trace elements. Rajasthan is a great place to find calcite with a high whiteness.
6) Calcite in Ceramics: Calcite from our company is used in a wide range of ceramic products, including art ware, porcelain, pottery, floor tiles, and sanitary ware, to name a few.
Structure and formula:
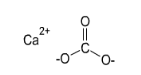
Calcium carbonate has the chemical formula CaCO3 and a molar mass of 100.1 g/mol. It’s a bivalent calcium cation (Ca2+) and a bidentate carbonate anion (CO32-), in which the carbon is connected to two oxygen atoms via single bonds and one oxygen atom via a double bond.
Occurrence:
Calcium carbonate can be found in a variety of mineral forms, including pure calcite, aragonite, and vaterite, as well as impure minerals including limestone, chalk, marble, and travertine. It’s also found in eggshells, seashells, oyster shells, snail shells, corals, and other materials.
Physical properties:
Pure CaCO3 is a fine white powder with no odour. Its calcite form has a density of 2.71 g/mL and a melting point of 1,339 °C. Aragonite, the other typical mineral form, has a density of 2.83 g/mL and a melting temperature of 825 °C.
Calcium carbonate is a chemical compound that is insoluble in water and stable at room temperature. When heated to high temperatures, it decomposes, releasing carbon dioxide and forming calcium oxide.
CaCO3+ CO2 = CaO
When CaCO3 reacts with acids, it also produces carbon dioxide.
Calcium carbonate forms the water soluble calcium bicarbonate salt (Ca(HCO3)2) when it combines with carbon dioxide-containing water.
Solubility:
Calcium Carbonate is sparingly soluble in water, with a solubility of 47 mg/L under normal circumstances. Calcium Bicarbonate, on the other hand, is much more soluble, and when calcium carbonate is exposed to water, it transforms into calcium bicarbonate, which is somewhat more soluble.
Conclusion:
Calcium carbonate makes up more than 4% of the earth’s crust. As a result, calcite, aragonite, and vaterite, three calcium carbonate minerals, are among the most essential rock-forming minerals. Nature’s calcium carbonate deposits are not limited to rocks; nearly all bodies of water, as well as many plants and animals, contain significant amounts of the mineral. The calcium carbonate cycle connects these natural resources.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






