Phosphoric acid (H3PO4), also referred to as orthophosphoric acid, is the most important oxygen acid of phosphorus and is used to generate fertiliser phosphate salts. It’s also employed in dental cements, albumin derivative manufacturing, and the sugar and textile sectors. It is used in food goods as an acidic, fruity flavouring.
Phosphoric acid is a crystalline solid with a melting point of42.35℃; in less concentrated form, it is a colourless syrupy liquid with a melting point of of 42.35℃; Phosphate rock is used to make crude acid, while white phosphorus is used to make higher grade acid.
Phosphoric acid
Phosphoric acid (H3PO4) is an odourless and colourless inorganic acid which contains phosphorus. It has 4 oxygen atoms, 1 phosphorus atom, and 3 hydrogen atoms. It occurs as a transparent crystalline solid or a clear colourless liquid. The pure solid has a melting point of of42.35℃ and a density of 1.834 g/cm³. The liquid is an aqueous solution with a concentration of85%. Phosphoric (V) acid or Orthophosphoric acid are other names for it.
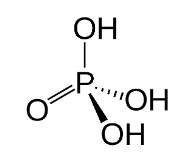
Structure of Phosphoric Acid
The structure of Phosphoric Acid is made up of a primary Phosphorus atom that is linked to an oxygen atom by a double bond. A single connection connects it to 3 hydroxyls as well.
Properties
The physical and chemical properties of phosphoric acid are listed below:
Physical Properties
Phosphoric acid has the following physical properties:
It is a colourless, odourless, viscous liquid with a high degree of red litmus property and a density of 1.834 g/cm³.
- It has a melting point of 42.35℃ and is white and crystal-like whenever solid.
- It is naturally non-toxic and non-volatile.
- If subjected to red heat and cooled, it creates a clear brittle glass.
- While heated, the acid preserves its original composition while gaining new properties.
Learn About the Chemical Reactivity of Phosphoric Acid with Oxygen
Chemical Properties
Phosphoric acid has the following chemical properties:
It can release up to three hydrogen ions, allowing it to react differently than mineral acids.
By substituting 1, 2, or 3 hydrogen atoms in a reaction with bases, 3 types of salts are formed. Separately, Na2HPO2, NaHPO2and NaPO2are produced.
As observed in Polyphosphoric and Meta Phosphoric acids, whenever molecules are subjected to high temperatures, they form dimers, trimers, and lengthy polymeric chains.
Phosphates
Phosphates are phosphoric acid salts. Phosphoric acid salts are chemicals which are widely employed in agriculture, industrial, and household application. Phosphoric acid contains 3 key phosphates:
Ammonium Phosphate
Monoammonium dihydrogen phosphate and diammonium hydrogen phosphate are the most prevalent phosphate salts. They are used in fertilizer and are made by mixing the right amount of phosphoric acid and anhydrous ammonia in a rotating drum. The quantity of nitrogen and phosphorus necessary for the crop determines which ammonium phosphate to use.
Calcium Phosphates
Calcium phosphates are also commonly employed in the field of fertilisers. Superphosphate is another term for them. Sulphuric acid reacts with phosphate rock to produce calcium dihydrogen phosphate.
Sodium Phosphates
Sodium phosphates are generated whenever phosphoric acid combines with a concentrated solution of sodium hydroxide under specified conditions and quantities. The end result is a solid crystal product. The following are some instances of sodium phosphates:
Monosodium dihydrogen phosphate is a chemical compound which is commonly used in metal cleaning and surface formulation. Also used as a pH control ingredient in toothpaste and as a sanitary ware enamel coating. It could also be used to coat lead pipes to keep them from dissolving due to the acids in the water.
Disodium hydrogen phosphate is a softening agent used in cheese, enamels and ceramic glazes, dye manufacturing, and other applications.
Trisodium phosphate is a chemical which is used in heavy-duty cleansers, degreasing steel, and other applications.
Disodium pyrophosphate is a food additive that is used to make bread and pastries. Also used in oil-well boring mud as a dispersion.
Phosphoric acid, food grade: used to acidify foods and beverages.
Preparing Phosphoric Acid
There are two ways to make phosphoric acid: wet process and heat process.
Wet Process to Prepare Phosphoric Acid
Phosphoric acid is generated in the wet method from fluorapatite, a crystal rock which includes the phosphate mineral. Concentrated sulfuric acid and water are used to react the chemical. The reaction produces phosphoric acid and sulphate, as well as several additional contaminants. Filtration and evaporation are used to eliminate surplus chemical compounds and contaminants. This procedure produces impure acid, which is usually employed in fertilisers without additional purification.
Thermal Process to Prepare Phosphoric Acid
The phosphorus is heated or burned at a high temperature in the presence of air in this chemical reaction. Phosphorous pentoxide is produced as a result of the burning or heating, and it is condensed to form a white powder. Phosphoric acid is made by hydrating this white powder in a separate procedure.
formula of phosphoric acid
The formula of phosphoric acid is given as:
H3PO4
phosphoric acid and oxygen
Phosphoric acid (H3PO4), also referred to as orthophosphoric acid, is the most important oxygen acid of phosphorus and is used to generate fertiliser phosphate salts. It’s also employed in dental cements, albumin derivative manufacturing, and the sugar and textile sectors.
Uses of Phosphoric Acid
Phosphoric acid is a widely utilised chemical component in a variety of sectors, including food production. The following are some of the most well-known applications of phosphoric acid:
Agriculture
It is used in the production of fertilisers as well as in animal and poultry feed as a flavouring additive.
Dentistry
It’s used as an etching solution and for tooth cleaning in dentistry. It’s also utilized in various mouthwashes.
Treating Rust
Phosphoric acid is employed in phosphate conversion coatings to prevent corrosion. It’s also used to remove rust from metal compositions and cure it.
Skincare Products
It’s employed in skin care products to keep the pH balanced. It’s in toothpaste, soaps, and detergents, among other things.
Food Processing
It’s used as a food ingredient as well as to make food and beverages more acidic. It’s also employed in the dairy industry.
Conclusion
Phosphoric acid is an acid with 4 oxygen atoms, 1 phosphorus atom, and 3 hydrogen atoms. It’s also described as phosphoric(V) acid or orthophosphoric acid. It’s found in teeth and bones, and it helps in metabolic processes. The salts of phosphoric acid are classified as phosphates. Phosphoric acid salts are compounds that are commonly used in agriculture, industry, and domestic use. The formula of phosphoric acid is given as:
H3PO4
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






