Tautomerism is a phenomenon where a single chemical compound tends to exist in two or more interconvertible structures that are completely different in terms of the relative position of one atomic nucleus which is generally hydrogen. The two structures are called tautomers. These types of isomer compounds usually differ only in the number of electrons and protons and they also exist in dynamic equilibrium.
When there is a reaction between these compounds only the transfer of protons takes place. tautomerism is also called desmotropism.
Tautomerism mostly takes place in the presence of a catalyst.
- Acid-catalyst: Here firstly the protonation occurs, the cation will be delocalized, Then, deprotonation will occur in the adjacent position of the cation.
- For base catalysts: deprotonation is the first step. Here, instead of cation delocalization, anion delocalization occurs and finally protonates to the different position of that anion.
Tautomerism Example:
If we consider the simple definition of tautomerism then it is described as a type of isomerism wherein the isomers interchange between one another very easily in order to exist together in equilibrium. During the reaction, proton transfer occurs in an intramolecular fashion.
Ketone-enol, enamine – imine, lactam-lactim, etc are some of the best examples of tautomers. Meanwhile, some of the key features of tautomerism are that this process gives more stability for the compound than others. In this phenomenon, there is an exchange of a hydrogen atom between two other atoms while forming a covalent bond to either one of the carbon atoms of two. Note:- Tautomerism is a reversible process.
Structural Requirement of Tautomerism:
- The Compounds contain polar molecules and weakly acidic functional groups.
- It involves the change in position of one atom with another.
- It poses effects on bond length or such features.
- It mostly occurs in planar or non-planar molecules.
Tautomerism Types:
In 1880 a scientist named Emil Erlenmeyer developed rules for tautomerism. He was one of the first scientists to have studied keto-enol tautomerism. This rule states that the hydroxyl group in all alcohols is attached to a double-bonded carbon atom directly, and gives ketones or aldehydes. This occurred due to the high stability of the keto form.
There are different types of tautomerism but among them, the keto-enol tautomerism is the most important one. In this form, one structure is a ketone and the other one is in enol form. Both of the tautomeric forms are interconvertible to each other by the use of either acid or base catalysts. This process of conversion of the ketone to enol is also known as enolization.
- Prototropy
- Annular Tautomerism
- Valence Tautomerism
- Tautomerism in Non-Carbonyl Compounds
- Tautomeric Form of Unsymmetrical Ketones
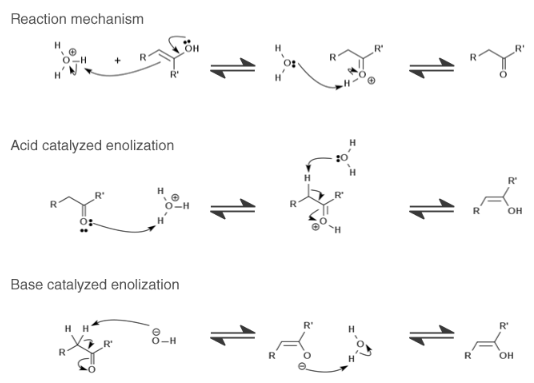
- Tautomerism Reaction Mechanism
Tautomerism Reaction Mechanism:
Now Let’s discuss the acid catalysis of keto-enol tautomerization. It is a two-step process done in an aqueous solution of acid. The carbon atom closest to the functional group is known as the alpha carbon atom. So, for this mechanism to happen at least one hydrogen atom must be with the alpha carbon atom. It is also known as an alpha hydrogen atom.
The hydrogen atom is added parallelly to the antibonding pi-orbital of the carbonyl group. This bond will undergo hyperconjugation with the C-H bond and reduce the electron density at the alpha carbon atom, where the alpha hydrogen atom will become more acidic than earlier. If the position of alpha hydrogen is not then the process of tautomerism will be very slow. Adamatanone is an example of this slow process.
In this process, we should follow Markovnikov’s rule for the additional part. Firstly, in the mechanism, there is a hydronium ion (H3O+) present which is an electrophile, therefore the electrons exposed near the C=C bond will be donated. If the no. of hydrogen atoms present in the compound is more, then the addition of protons also increases.
Propyne
- Formula: C3H4
- Molecular weight: 40.0639
- IUPAC Standard InChI: InChI=1S/C3H4/c1-3-2/h1H,2H3
- IUPAC Standard InChIKey: MWWATHDPGQKSAR-UHFFFAOYSA-N
- CAS Registry Number: 74-99-7
- Chemical structure:
- Isotopologues:
- Propyne-3,3,3-d1
- Propyne-d4
- Other names: Methylacetylene; 1-Propyne; Allylene; Propine; CH3C≡CH; Acetylene, methyl-
Allenes:
The Allenes are organic compounds in which one carbon atom has a double bond with each of its two adjacent carbon centers. The Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is also called allene. Compounds with an allene-type structure but with more than three carbon atoms are the members of a larger class of compounds called cumulenes with X=C=Y bonding.
Formula for Allene is | C3H4
The Allene compounds are unique chiral synthons devoid of chirality centers and have been used for the syntheses of various chiral compounds.
Conclusion-
Here in this article, we have discussed tautomerism. We now know that tautomers are the isomers of a compound that differ only in the position of the protons and electrons. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism. Structural Requirement of Tautomerism has also been discussed. Different types of tautomerism and Tautomerism reaction mechanisms have been talked about in detail. We have gone through propyne and allenes. The Allenes are organic compounds in which one carbon atom has a double bond with each of its two adjacent carbon centers. They are classified as cumulated dienes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




