The chemical compound hydrogen peroxide has the formula H2O2. It’s a very pale blue liquid that’s somewhat more viscous than water in its purest state. It’s used as an oxidant, bleaching agent, and antiseptic in a weak solution (3–6% by weight) in water for consumer use, and at larger concentrations for industrial usage. When heated, concentrated hydrogen peroxide, often known as “high-test peroxide,” decomposes explosively and has been employed as a rocket propellant.
The simplest peroxide, hydrogen peroxide, is a reactive oxygen species with a single oxygen–oxygen link. When exposed to light, it decomposes slowly, but quickly in the presence of organic or reactive substances. It’s usually kept in a dark bottle with a stabiliser in a mildly acidic solution to keep light out. The human body contains hydrogen peroxide, which is found in biological systems. Peroxidases are enzymes that utilise or breakdown hydrogen peroxide.
Structure
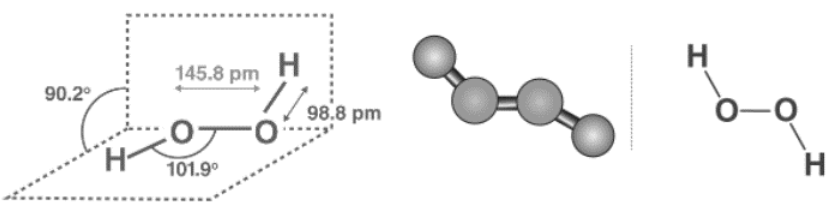
Paul-Antoine Giguère used infrared spectroscopy to demonstrate that hydrogen peroxide (H2O2) is a nonplanar molecule with (twisted) C2 symmetry for the first time in 1950. Despite the fact that the O-O bond is a single bond, the molecule has a relatively high rotational barrier of 386 cm-1 (4.62 kJ/mol) in the trans configuration and 2460 cm-1 (29.4 kJ/mol) in the cis configuration for rotation between enantiomers. These barriers are thought to be caused by repulsion between neighbouring oxygen atoms’ lone pairs and dipolar interactions between the two O–H bonds. The rotational barrier for ethane is 1040 cm-1 (12.4 kJ/mol).
The molecule is chiral due to the nearly 100° dihedral angle between the two O–H bonds. Enantiomers occur in the smallest and most basic molecules. It has been suggested that enantiospecific interactions of one enantiomeric form of ribonucleic acids led to multiplication of one enantiomeric form of ribonucleic acids and hence an origin of homochirality in an RNA universe.
Physical properties of hydrogen peroxide
- In its purest form, hydrogen peroxide is virtually colourless (very pale blue).
- Its boiling point has been calculated at 150.2 degrees Celsius, which is over 50 degrees Celsius more than the boiling point of water.
- Hydrogen Peroxide has a melting point of -0.43 C.
- In water, it produces hydrates and makes a homogenous mixture in all amounts.
- Hydrogen Peroxide has a molar mass of 34.0147 g/mol.
- It has a somewhat acidic odour.
- In aqueous solution, it has a density of 1.11 g/cc, whereas in pure form, it has a density of 1.450 g/cc.
- Hydrogen peroxide dissolves in ether, alcohol, and petroleum ether but not in petroleum ether.
Chemical properties of hydrogen peroxide
In both acidic and basic mediums, hydrogen peroxide serves as an oxidising and reducing agent.
1. Oxidising nature of hydrogen peroxide in acidic medium:
PbSs+4H2O2aq→PbSO4s+4H2O(l)
2. Reducing nature of hydrogen peroxide in acidic medium:
HOCl+H2O2H3O++Cl–+O2
3.Oxidising nature of hydrogen peroxide in basic medium:
Mn2+H2O2→Mn4+2OH–
4.Reducing nature of hydrogen peroxide in basic medium:
I2+H2O2+OH–→2I–+2H2O+O2
Uses of hydrogen peroxide
- In the textile and paper industries, hydrogen peroxide is utilised as a bleaching agent. According to data, nearly 60% of the world’s Hydrogen Peroxide output is utilised for pulp and paper bleaching.
- In everyday life, hydrogen peroxide may be used as a hair bleach as well as a light disinfectant.
- Organic compound production: Hydrogen Peroxide is used to make a variety of organic Peroxides, with benzoyl Peroxide being one of the most common. It’s utilised in polymerizations, as well as as a flour bleaching agent and an acne treatment.
- Hydrogen peroxide is also used to eliminate organic pollutants in some waste-water treatment systems.
- Hydrogen peroxide is used to sterilise a variety of surfaces, including surgical instruments, and it may also be used to sterilise rooms as a vapour (VHP).
- Because it degrades to water and oxygen, hydrogen peroxide is a safer alternative to chlorine-based bleaches for the environment. It is also generally acknowledged as safe as an antibacterial agent by the US Food and Drug Administration (FDA).
Conclusion
At room temperature, hydrogen peroxide is a colourless liquid with a harsh flavour. In the air, small levels of gaseous hydrogen peroxide exist naturally. With the release of heat, hydrogen peroxide decomposes quickly to oxygen and water. It is a potent oxidising agent that can generate spontaneous combustion when it comes into touch with organic material, despite being non-flammable. Many houses have modest quantities of hydrogen peroxide (3-9 percent) for medical purposes and as a clothing and hair bleach. Higher quantities of hydrogen peroxide are used in industry as a bleach for textiles and paper, as a constituent of rocket fuels, and for the manufacture of foam rubber and organic compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




