A hydrocarbon is a type of organic compound that is composed solely of carbon and hydrogen molecules. A hydrocarbon is the most basic type of organic molecule, and it serves as the building block for all other more complex organic compounds. Aromatic hydrocarbons are distinguished from aliphatic hydrocarbons, which are distinguished by their lack of aromaticity. In the case of aliphatic hydrocarbons, this means that they do not contain the benzene group or the benzene ring. Aromatic hydrocarbons are organic compounds that contain one or more benzene rings. In this section, we will discuss aliphatic hydrocarbons in greater detail.
Alkanes
An alkane is a hydrocarbon in which there are only single covalent bonds, as opposed to other hydrocarbons. Methane, which has the molecular formula CH4, is the most basic of the alkanes. The carbon atom is the central atom, and it forms four single covalent bonds with hydrogen atoms to form the compound. It is known as ethane (C2H6), and it is the next most basic alkane to be discovered. It is composed of two carbon atoms that form a single covalent bond between them. Each carbon atom is then capable of forming a three-way bond with three hydrogen atoms. Starting from there, the alkane series progresses, each time lengthening the length of the carbon chain by one carbon at a time. propane (C3H8), and butane are all represented by structural formulas (C4H10).
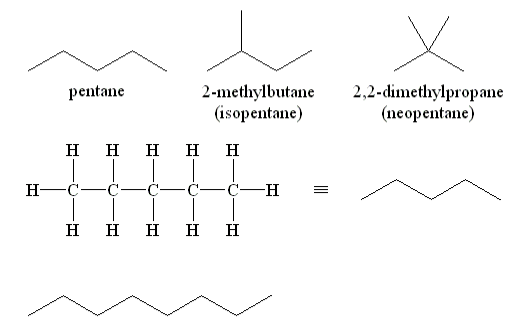
Due to the fact that the carbon atoms are linked together in a single continuous chain with no branches, these alkanes are known as straight-chain alkanes. Straight-chain alkanes are easy to name and write structural and molecular formulas for because they have a simple structure. The name of each alkane is made up of a prefix that specifies the number of carbon atoms in the molecule and the suffix -ane at the end. The molecular formula follows the pattern of CnH2n2, where n denotes the number of carbon atoms in the chain of molecules.
A condensed structural formula is a type of structural formula that is a variation of the original structural formula. Covalent bonds are understood to exist between each carbon atom and the hydrogens that are associated with it, as well as between carbon atoms themselves, according to this formula. Aside from that, this table demonstrates that the boiling points of the alkanes steadily increase as the length of the carbon chain is increased. In other organic molecules, this is due to an increase in the strength of the intermolecular attractive forces, and it is a characteristic that is common to all organic molecules.
In summary, a hydrocarbon is an organic compound that is composed solely of carbon and hydrogen; it is the most basic type of organic molecule.
In the case of aliphatic hydrocarbons, this means that they do not contain the benzene group or the benzene ring.
Aromatic hydrocarbons are organic compounds that contain one or more benzene rings.
An alkane is a hydrocarbon in which there are only single covalent bonds, as opposed to other hydrocarbons.
What is the Simplest Straight-Chain Alkane?
Inorganic compound composed solely of carbon and hydrogen, called a hydrocarbon alkane.
Alkanes are saturated hydrocarbons that contain only single covalent bonds.
a saturated hydrocarbon with any number of carbon atoms arranged one after another in a chain straight-chain alkanes
A homologous series is a collection of compounds in which the amount of change in molecular structure from one compound in the series to the next is constant from one compound in the series to the next.
When some bonds and/or atoms are left out of a structural formula, the presence of these atoms or bonds is understood to exist.
A substituent is an atom or group of atoms that can be used to replace a hydrogen atom on a hydrocarbon molecule that is not its parent.
group is a hydrocarbon substituent; the methyl group (—CH3) is an alkyl group
Alkane with one or more alkyl groups attached to the parent structure is known as a branched-chain alkane.
What is the relationship between the number of valence electrons in carbon atoms and the number of bonds formed by carbon atoms?
Because carbon atoms have four valence electrons, they always form four covalent bonds when they are combined.
In an alkane, what are the two possible arrangements of carbon atoms?
The carbon atoms in an alkane can be arranged in a straight chain or in a chain with branches, depending on the type of alkane.
Straight-Chain Alkanes Examples
Conclusion
Alkanes, alkenes, alkynes, and aromatic compounds are the four main types of hydrocarbons. The answer to the question “what are alkanes?” is that they are saturated acyclic/aliphatic hydrocarbons. Open-chain compounds (branched or unbranched) are defined as those in which each carbon atom is only covalently linked to the other carbon atoms in the chain. Also known as paraffin, alkanes are hydrocarbons that are found in nature. There are two carbon atoms and one hydrogen atom in a tree-like structure, which is held together by a single covalent bond. Typically, alkanes are represented by the formula CHn+2. The properties of alkanes, as well as their variations, will be discussed here. This section describes the chemical structure of methane (CH4), which is one of the most abundant alkanes on Earth.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




