All alkanes have names that end in -ane. Whether the carbons are linked end-to-end in a ring (referred to as cyclic alkanes or cycloalkanes) or contain side chains and branches, the name of any carbon-hydrogen chain that does not have any double bonds or functional groups would then end with the suffix –ane The total number of carbons in an unbranched carbon chain is used to name alkanes.
A single chemical compound can have multiple acceptable systematic names, and the systematic approach to naming organic chemical compounds is known as IUPAC Nomenclature; however, no two compounds can have the same name.
IUPAC Nomenclature
For the naming of compounds, the IUPAC (International Union for Pure and Applied Chemistry) established a common naming system based on standard rules. This is referred to as IUPAC naming or IUPAC nomenclature.
Nomenclature One major distinction between chemistry’s language and other scientific and natural languages is the way things are named. The other difference is the significance of written language versus spoken language. In chemistry, not only elements and compounds should be named, but also reactions, methods, pieces of apparatus, and theoretical concepts.
Alkanes
Alkanes are the most basic hydrocarbons we know. Their general formula is CnH2n+2. Alkanes are a type of saturated hydrocarbon; they have only sigma bond linkages between a carbon and a hydrogen. The organic compounds form a series known as the homologous series, in which the subsequent compounds share the same functional group but differ by a ‘–CH2’ group.
A large number of organic compounds are made up of alkanes . Many organic compound names are based on alkanes as a result of these considerations.. Furthermore, many vital components of organic molecules involve one or more alkyl groups bonded as substituents onto the basic organic molecule, minus a hydrogen atom. As a result of these factors, many organic compound names are based on alkanes.
Drawing Hydrocarbons
It can take a long time to write out each atom and link manually when drawing hydrocarbons. A skeletal structure is a shorthand symbol used in organic chemistry to depict hydrocarbons. Only the bonds between carbon atoms are depicted in a skeletal form. Individual carbon and hydrogen atoms, as well as
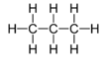
hydrogen bonds, are not drawn. When a molecule only has single bonds (sp3 bonds), these bonds are shown in a “zig-zag” pattern. This is due to the fact that all bonds in the tetrahedral geometry point as far apart as feasible, and the structure is not linear. Consider the following diagrams of the propane molecule:
It’s worth noting that hydrogens aren’t represented in skeletal structures. In the lack of double or triple bonds, carbon forms four total bonds, implying the presence of hydrogens. When the structure specifies an insufficient number of bonds to a carbon atom, the rest of the bonds are assumed to be made to hydrogens.
It is acknowledged that there are carbon atoms (with h3 joined ) at the terminal ends of the structure, but only the bonds are being dragged out.
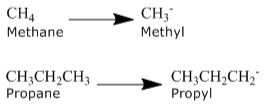
Alkyl Groups
The formula CnH2n+2 can be used to characterise alkanes. The formula CnH2n+1 describes an alkyl group generated by removing one hydrogen from an alkane chain. When this hydrogen is removed, the stem changes as -ane to -yl. Take a look at the samples provided below.
Alkanes Names
Name | Molecular Formula | Condensed Formula |
Methane | CH4 | CH4 |
Ethane | C2H6 | CH3 CH2CH3 |
Propane | C3H8 | CH3 (CH2 )2CH3 |
Butane | C4H10 | CH3 (CH2 )3CH3 |
Pentane | C5H12 | CH3 (CH2 )4CH3 |
Hexane | C6H14 | CH3 (CH2 )5CH3 |
Heptane | C7H16 | CH3 (CH2 )6CH3 |
Octane | C8H18 | CH3 (CH2 )7CH3 |
Nonane | C9H20 | CH3 (CH2 )8CH3 |
Decane | C10H22 | CH3 (CH2 )9CH3 |
Undecane | C11H24 | CH3 (CH2 )10CH3 |
Dodecane | C12H26 | CH3 (CH2 )11CH3 |
Tridecane | C13H28 | CH3 (CH2 )12CH3 |
Tetradecane | C14H30 | CH3 (CH2 )13CH3 |
Pentadecane | C15H32 | CH3 (CH2 )14CH3 |
- IUPAC Alkane Nomenclature Rules:
- Find the longest continuous carbon chain and identify it.
- Identify and name the groups that are linked to this chain.
- Work your way down the chain, starting at the end closest to a substituent group and counting down. Assign the location of each substituent group with a number and a name.
- Assemble the name by listing groups alphabetically.
- When alphabetizing, the prefixes di, tri, tetra, and so on, which are used to assign several groups of the same kind, are not taken into account.
- Three Naming Principles:
- Choose the carbon chain with the most substituents and the longest length that contains a functional group.
- A carbon bonded to a functional group have to have the lowest carbon number possible. When there are neither any functional groups, then any substitute must have the fewest number of substituents possible.
- Consider the alphabetical order; that is, after applying the first two rules listed above, ensure that their substitutes and/or functional groups are marked in alphabetical order.
Conclusion
An alkane, often known as paraffin (a historical term with several meanings), is an acyclic saturated hydrocarbon in organic chemistry. To put it another way, an alkane is made up of hydrogen and carbon atoms organised in a tree structure with single carbon–carbon bonds. CnH2n+2 is the typical chemical formula for alkanes. The alkanes range in complexity from the simplest example of methane (CH4), where n = 1 (often referred to as the parent molecule), to arbitrarily big and complicated compounds, such as pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, a tetradecane isomer (C14H30).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






