The study of electricity and how it affects chemical processes is known as electrochemistry. Electricity is created in electrochemistry by the passage of electrons from one element to another in a redox or oxidation-reduction process.
In biological systems, electrochemistry plays a crucial role in the transmission of nerve impulses. All electrochemical processes are based on redox chemistry, or the transfer of electrons. Any device that turns chemical energy into electrical energy or electrical energy into chemical energy, such as the batteries we use every day, is an electrochemical cell.
Brief comprehension about redox reactions and electrochemistry-
Now we will learn more about what we mean by electrochemistry, redox reactions, unusual oxygen oxidation states, disproportionation, and finally at redox titration.
Electrochemistry-
Electrochemistry is the study of chemical reactions that occur in a solution at the interface of an electron conductor and an ionic conductor. Electron transfer occurs between the electrode and the electrolyte or species in solution in these reactions. An electrochemical reaction is one in which a chemical reaction is driven by an externally applied voltage, such as electrolysis, or one in which a voltage is generated by a chemical reaction, such as in a battery. Oxidation/reduction processes, on the other hand, are chemical reactions in which electrons are transported between molecules. Electrochemistry, in general, is concerned with circumstances in which oxidation and reduction processes are separated in space or time and are linked by an external electric circuit.
Redox reactions-
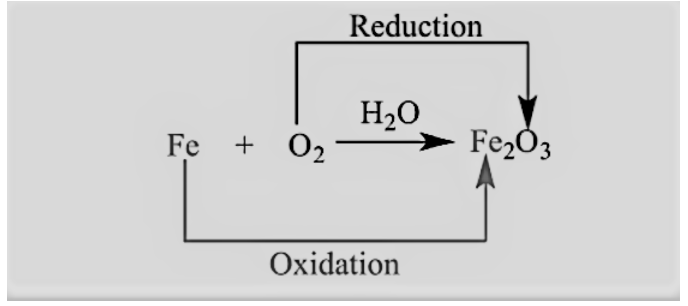
A change in the oxidation state of two atoms determines a redox reaction. There is no redox reaction if the oxidation number does not change.
It’s a kind of chemical reaction where two species trade electrons. An oxidation-reduction process is a chemical reaction in which a molecule, atom, or ion changes its oxidation number by gaining or losing an electron.
Redox reactions have two halves: a reduced half and an oxidized half that always happen at the same time. As the reduced half acquires electrons, the oxidation number decreases, whereas the oxidized half loses electrons as the oxidation number increases.
An oxidizing agent is an ion or molecule that accepts electrons and then uses them to oxidize other species and a reducing agent is an ion or molecule that transfers electrons, lowering the number of other species in the process.
Unusual oxygen oxidation states-
Now firstly, we will see what do we mean by oxidation state then we will cover unusual oxygen oxidation states, so, the potential to acquire, give, or share electrons is reflected in the oxidation-reduction reaction’s oxidation number. The iron ion Fe3+ has an oxidation state because it may acquire three electrons to form a chemical bond. number of +3, while the oxygen ion O2 has an oxidation number of 2 because it can contribute two electrons. The sum of the oxidation numbers in an electrically neutral material is zero; for example, the oxidation number The oxidation number of the three oxygen atoms (6) is balanced by the two iron atoms (+6 total) hematite (Fe2O3).
Now coming onto unusual oxygen oxidation states – C-H bond activation and O-O bond creation are two essential reactions that high oxidation state metal centres execute in nature. Transition metal ions in the 4+ oxidation states are also often mentioned as intermediates in a variety of catalytic reactions, including water oxidation. Due to their reactive and frequently highly oxidizing character, isolation of these intermediates has proved problematic, this causes unusual oxidations states or we can say this is what we mean by unusual oxygen oxidation states.
Disproportionation–
Disproportionation reaction- This is a reaction in which the same element is oxidized and reduced at the same time. Such reactions occur when one of the reactive elements has at least three oxidation states.
In addition, the reactive molecule’s element must be in the intermediate oxidation state, while oxidation and reduction need the lower and higher oxidation states, respectively. A common example of such a process is the breakdown of hydrogen peroxide, in which oxygen species are disproportionated.
Mn2O3 becoming Mn2+ and MnO2
Redox titration-
Now we will first see what do we mean by titration- Titration is a laboratory method for determining the concentration of an unknown solution using a solution of known volume and concentration. Between the two solutions, an oxidation-reduction process or acid-base neutralization occurs, and the known numbers are utilized to compute the unknown. The titrant or titrator refers to the known concentration standard solution, while the titrant or analyte refers to the unknown concentration solution. Titrimetry is another name for titration.
Coming onto redox titration-
Redox titration is an analytical technique for determining the concentration of a supplied analyte by initiating a redox reaction between a titrant and an unknown strength provided analyte. These types of titrations may need the use of a potentiometer or a redox indicator.
Example: Using a starch indicator to aid identify the endpoint, treating an iodine solution with a reducing agent to make iodide.
Redox titration may also be used to estimate the concentration of an unknown solution (analyte) containing an oxidizing or reducing agent. An external indicator isn’t required for all titrations. When potassium permanganate is titrated against a colourless analyte, certain titrants may act as their own indicators.
Conclusion-
In this article we read about electrochemistry, redox reactions, unusual oxidation states, disproportionation, titration and finally at redox titration. Electrochemical cells may be found in a variety of places in our daily lives, from throwaway AA batteries in remote controls to lithium-ion batteries in phones to nerve cells scattered throughout our bodies. Galvanic and electrolytic cells are the two kinds of electrochemical cells. Electrochemistry is also engaged in the manufacture of materials by electrorefining or electrodeposition, as well as the corrosion of materials, same goes for redox reactions, it has its importance in our bodily functions and as well as industrial uses too.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




