Basic phosphorus occurs in two major forms-
- White phosphorus
- Red phosphorus
It is highly reactive, phosphorus is never found as a free component on Earth.Red Phosphorus has a concentration in the Earth’s crust of about 1 g/kg. In minerals, phosphorus naturally exists as phosphorus. First, understand what is Red Phosphorus?
Red phosphorus is one of the most familiar allotropes of phosphorus. It is contemplated to be a by-product of the P4 molecule. It prevails in an amorphous, i.e., a non-crystalline web of phosphorus atoms. It is found to be more stable than white phosphorus which is another generally occurring phosphorus allotrope. Red phosphorus is distinguished by its colour deep red with a powdery texture.
White phosphorus withstands a gradual transformation to produce the red phosphorus allotrope. This change arises quicker in the existence of light and energy in the form of heat. When a sample of white phosphorus is partly converted into red phosphorus, it determines a characteristic yellow colour.
Red Phosphorus: Overview
Red phosphorus exists in a polymeric bond of tetrahedral structure P4 molecules in which one of the P-P bonds are split to enable the linking of these tetrahedrons.The red phosphorus diagram is given below:
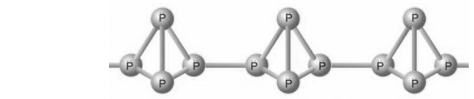
From the red phosphorus diagram given above, it can be examined that the pattern of red phosphorus is relatively comparable to that of a P4 molecule. Each phosphorus atom in P4 is associated with three other phosphorus atoms in a tetrahedral pattern. When one of these bonds is broken, these tetrahedral configurations can proceed to bond with neighbouring phosphorus atoms, happening in a polymer-like configuration.
Properties of Red Phosphorus
A few important physical and chemical properties of red phosphorus are tabulated below –
Physical Properties of Red Phosphorus
The physical properties of red phosphorus are discussed below:
- Molar Mass = 30.974 grams per mole
- Melting point = 860K
- Density = 2.34 grams per cubic centimetre
- Red phosphorus formula = P4 (chain)
Chemical Properties of Red Phosphorus
The chemical properties of red phosphorus are listed below:
- The chemical properties of red phosphorus are discussed below:
- Red phosphorus is odourless.
- It has a deep red colour.
- It is not poisonous to humans in comparison to the white phosphorus allotrope.
- Heating to temperatures above 300oC, red phosphorus undergoes crystallization.
- It can also determine a cubic configuration in its crystal lattice.
- This allotrope of phosphorus does not show phosphorescence, i.e., type of photoluminescence.
- Red phosphorus is not chemically reactive as compared to its white phosphorus companion.
Difference between Red Phosphorus and White Phosphorus
The difference between red phosphorus and white phosphorus are illustrated below-
- Red phosphorus is an allotrope of phosphorus that has a dark red colour whereas white phosphorus is an allotrope of phosphorus that exist as a translucent waxy solid.
- Red phosphorus exists as a polymeric network but white phosphorus exists as P4 molecules.
- Red phosphorus appears as dark red coloured crystals whereas white phosphorus exists as a translucent waxy solid that quickly becomes yellow when exposed to light.
- Red phosphorus is nontoxic and less reactive but white phosphorus is toxic and highly reactive.
- Red phosphorus is not phosphorescent but white phosphorus is phosphorescent.
- Red phosphorus ignites in the air at temperatures above 240°C whereas white phosphorus ignites air at low temperatures such as 50°C.
- This is produced via heat-treating white phosphorus where this is produced via heating phosphorus rock in an electric or fuel-fired furnace, i.e., in the presence of carbon and silica.
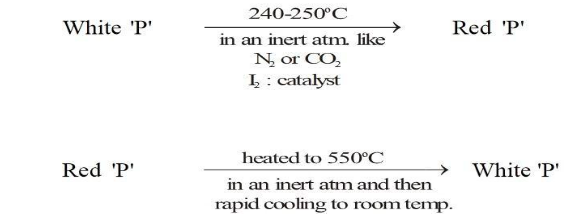
How to Turn Red Phosphorus into White Phosphorus?
We have come to know about red phosphorus but do you know how to turn red phosphorus into white phosphorus? To turn red phosphorus into white phosphorus a few different fundamental procedures to obtain white phosphorus are discussed in this subsection.The manufacture of white phosphorus involves the heating of red phosphorus at 550°C. In the presence of an inert atmosphere like N2 or CO2. Then there is a rapid cooling to room temperature to form a white phosphorus.
Applications of Red Phosphorus
The surface of a matchbox is composed of red phosphorus and powdered glass. This mixture can be utilized to produce a spark that can light a matchstick. A few other significant uses of red phosphorus are given below –
- Various flares that are used as emergency signals aim at this allotrope of phosphorus to enable the ignition process. The sustained combustion of the flare is also obtained with the help of this allotrope.
- When mixed with magnesium and a binder, red phosphorus can be used as a smoke device that can rapidly generate a smokescreen.
- It is also used in the making of methamphetamine normally known as meth.
- Red phosphorus is also used as a flame retardant in several thermoplastics and thermosetting plastics.
Conclusion
Hence, it can be stated as phosphorus is a chemical component that is represented by the symbol P which has the atomic number 15. Basic phosphorus is of two types, one is red phosphorus and another one is white phosphorous. Red phosphorusis highly reactive and is never found as a free component on earth and is one of the most famous allotropes phosphorous. Whereas the white phosphorus is another generally occurring phosphorous. Red phosphorus is not phosphorescent whereas white phosphorus is phosphorescent and ignites air at low temperature. Red phosphorus is used in day to day life which comprises of the surface of match box which is a mixture of red phosphorus and glass powder. This mixture can be utilized to produce a spark that can light a matchstick
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




