Ethylene glycol serves as an antifreeze in cooling and heating systems, as well as a solvent and hydraulic braking fluid. Acute (short-term) ingestion of significant amounts of ethylene glycol causes three stages of health impacts in humans: central nervous system (CNS) depression, cardiac effects, and renal impairment. In one trial of people who inhaled modest doses of ethylene glycol for nearly a month, the only side effects were throat and upper respiratory tract irritation. Chronic (long-term) exposure to ethylene glycol in the diet caused kidney damage and liver consequences in rats and mice.
Chemical Reactivity of Ethylene Glycol with Hydrogen
Hydrogen may be created using clean and renewable energy sources, and its oxidation produces 122 kJ/g of energy, which is 2.7 times more energy than ordinary hydrocarbon fuel combustion. It can be made from ethanol and glycols, both of which are obtained from cellulose fermentation or hydrolysis, assuring a renewable cycle. While aqueous phase reforming of glycols at high temperatures is feasible, however, because the required reaction energy is provided by burning fossil fuels, it may not be sustainable.. Photocatalytic hydrogen production utilising ultraviolet light may be more appealing because the cycle would be more sustained in this instance. Furthermore, because ethylene glycol and glycerol are commonly found in aqueous solution in petrochemical industrial waste streams and separation is difficult due to their hydrophilic character, these wastes can be used to produce photocatalytic hydrogen.
2OH+2e– → H2+2O
C2H6O2+ 2H2O→ 2CO2+5H2
C2H6O2+ 522O2 →2CO2+ 3H2O
Huber et al. outlined the ethylene glycol aqueous phase reforming processes, which produce adsorbed intermediates by cleaving C-C, C-H, and O-H bonds.
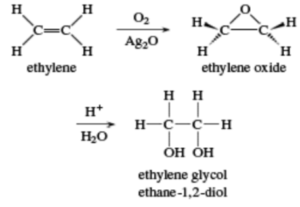
Structure of Ethylene Glycol
The simplest member of the glycol family of organic molecules is ethylene glycol, commonly known as ethane-1,2-diol. An alcohol having two hydroxyl groups on adjacent carbon atoms is known as a glycol (a 1,2-diol). The term ethylene glycol refers to a glycol that is generated from ethylene.
Ethylene glycol is a transparent, pleasant, slightly viscous liquid with a boiling point of 198 degrees Celsius (388.4 degrees Fahrenheit). A 1:1 solution of ethylene glycol and water boils at 129 degrees Celsius (264.2 degrees Fahrenheit) and freezes at 37 degrees Celsius (34.6 degrees Fahrenheit), making it an excellent coolant for vehicle radiators. Ethylene glycol is extremely dangerous, and animals or humans who consume it become extremely unwell and may die.
Ethylene Glycol and Hydrogen
Ethylene Glycol: Polyester fibres for garments, upholstery, carpet, and pillows; fibreglass used in items like jet skis, bathtubs, and bowling balls; and polyethylene terephthalate resin used in packaging film and bottles are all examples of ethylene glycol’s uses as a raw material. Many of these items are both energy and cost-effective, as well as recyclable.
The Ethylene Glycols Panel promotes the principles of Responsible Care and focuses on the generation, collection, evaluation, and dissemination of information related to the safe handling of ethylene glycols, as well as environmental and health issues arising from their production, storage, transportation, use, and disposal.
Hydrogen: Hydrogen is a clean fuel that produces just water when burned in a fuel cell. Natural gas, nuclear power, biomass, and renewable energy sources such as solar and wind power can all be used to produce hydrogen at home. It is a desirable fuel for transportation and energy generation because of these qualities. Automobiles, residences, portable power, and a number of other applications can all benefit from it.
Conclusion
Ethylene glycol has been shown to be fetotoxic in several trials of animals treated orally or by inhalation. Workers exposed to ethylene glycol did not have a higher risk of developing kidney cancer, according to an epidemiologic study. Ethylene glycol has not been designated as carcinogenic by the EPA.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






