Valence bond theory is concerned with electronic configuration, atomic orbitals (and their overlapping), and atomic orbital hybridization. Atomic orbitals overlapping and electrons being concentrated in the matching bond region generate chemical bonds. The electrical structure of molecules created by this overlapping of atomic orbitals is also explained by the valence bond theory. It also emphasises how one atom’s nucleus is attracted to the electrons of the other atoms in a molecule.
History Of Valence Bond Theory
The Lewis approach to chemical bonding failed to provide insight into how chemical bonds are formed. In addition, the valence shell electron pair repulsion theory (commonly known as VSEPR theory) had just a few applications (and also failed in predicting the geometry corresponding to complex molecules).
The valence bond hypothesis was proposed by German physicists Walter Heinrich Heitler and Fritz Wolfgang London to address these concerns. The Schrodinger wave equation was also used to explain the formation of a covalent bond between two hydrogen atoms. The valence bond hypothesis is used to depict the chemical bonding of two hydrogen atoms.
Postulates of Valence Bond Theory

The main postulates of the valence bond theory are listed below:
- When two valence orbitals (half-filled) from two separate atoms overlap on one other, covalent bonds are created. The electron density in the area between the two bonding atoms increases as a result of this overlapping, increasing the molecule’s stability.
- An atom’s valence shell has several unpaired electrons, allowing it to make many bonds with other atoms. The valence bond hypothesis states that the paired electrons in the valence shell are not involved in the formation of chemical bonds.
- Chemical bonds that are covalent are directed and parallel to the region corresponding to the overlapping atomic orbitals.
- Sigma bonds and pi bonds are distinguished by the pattern in which the atomic orbitals overlap, i.e. pi bonds are produced by overlapping along the axis containing the nuclei of the two atoms, whereas sigma bonds are formed by overlapping along the axis containing the nuclei of the two atoms.
Below is a diagram of how sigma and pi bonds are formed.
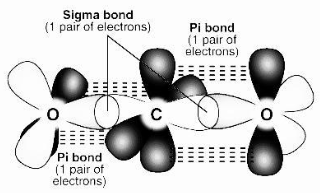
It’s worth noting that sigma bonds include atomic orbitals overlapping head-to-head, whereas pi bonds involve parallel overlapping.
Applications of Valence Bond Theory
- The creation of covalent bonds in many molecules can be explained by the maximum overlap requirement stated by the valence bond theory.
- One of its most essential applications is this. Discrepancies in overlapping orbitals can explain differences in the length and strength of chemical bonds in H2 and F2 molecules, for example.
- The overlap of the 1s orbital of the hydrogen atom and a 2p orbital of the fluorine atom forms the covalent link in an HF molecule, as explained by the valence bond theory.
Limitations of Valence Bond Theory
The valence bond theory has a number of flaws.
- The inability to explain the tetravalency of carbon.
- The energies of the electrons are not discussed.
- Electrons are thought to be confined in specific locations, according to the idea.
- It does not provide a quantitative assessment of coordination molecules’ thermodynamic or kinetic stabilities.
- There is no difference between weak and powerful ligands.
- There is no reason for the colour of coordination compounds.
Wave Function
A wave function is a mathematical representation of a quantum state of a particle as a function of momentum, time, location, and spin in quantum physics. The Greek letter psi, ψ is used to represent a wave function.
The likelihood of locating an electron within the matter-wave can be explained using a wave function. This can be achieved by incorporating an imaginary number that is squared to create a real number solution resulting in an electron’s position. With the help of the Schrodinger equation, the concept of wave function was introduced in 1925.
Schrodinger Equation
The linear partial differential equation defining the wave function is known as the Schrodinger equation. Erwin Schrodinger is the name of the equation. Schrodinger could work on the wave function using quantum mechanics postulates.
The Schrodinger equation is written as follows
HΨ = EΨ
H = Hamiltonian operator
Properties Of Wave Function
- The particle’s entire quantifiable information is available.
- It should be single-valued and continuous.
- Using the Schrodinger equation, calculating energy becomes simple.
- The wave function is used to create a three-dimensional probability distribution.
- The probability of detecting a particle is 1 if it exists.
Postulates of Quantum Mechanics
- The time evolution of the wave function is given using the time-dependent Schrodinger equation.
- Using the wave function, it is simple to understand a particle in a conservative field of force system.
- The set of eigenfunctions of operator Q is used to create a linear collection of independent functions.
- The Hermitian operator Q is connected with a physically observable property q.
- The expectation value of the property q can be obtained by conducting the expectation value integral with regard to the wave function associated with the system.
- There is an operator Q working on the wave function associated with a definite value of a physical observable such that it generates a wave function of that many times for every physical observable Q.
Excited State of An Atom
Valence electrons, which are present in the atom’s outermost occupied orbitals, may not necessarily stay in the same orbital or energy level because they can absorb energy from heat or light. When a valence electron absorbs enough energy, it reaches an excited state. The electron jumps from its original energy level or orbital, also known as the ground state, to an empty orbital of a higher energy shell that is further away from the nucleus. However, the excited electron does not remain in the excited state indefinitely, and eventually returns to the ground state.
Conclusion
The requirement of a maximum intersection, which hints at the establishment of the strongest conceivable bonds, is a key aspect of the Valence Bond theory. Many molecules employ this notion to describe the formation of covalent bonds. The Valence bond hypothesis is crucial because it aids in comprehending the concept of molecule bonding.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




