Pyrophosphoric acid, also known as diphosphate, is an inorganic compound having the formula H4P2O7 or more specifically [(HO) 2P (O)] 2O. Diethyl ether,water and ethyl alcohol are all soluble in it. The anhydride crystallizes into two polymorphs that melt at 54.3 ° C and 71.5 ° C. This compound is not particularly useful except that it is a component of polyphosphoric acid and a conjugate acid of the pyrophosphate anion. The anions, salts and esters of pyrophosphate are called pyrophosphate.
Preparation:
It’s best made by ion exchange from sodium pyrophosphate or hydrogen sulfide treatment of lead pyrophosphate. It is not made by dehydrating phosphoric acid. Instead, pyrroline acid is produced only as one of the products.
Reactions:
In the molten state, pyrophosphoric acid rapidly forms an equilibrium mixture of phosphoric acid, pyrophosphoric acid, and polyphosphoric acid. The weight ratio of pyrroline acid is about 40%, and it is difficult to recrystallize from the melt. Like all polyphosphoric acids, pyrophosphate hydrolysis in an aqueous solution, eventually establishing an equilibrium between phosphoric acid, pyrophosphate, and polyphosphoric acid.
H4P2O7 + H2O ⇌ 2H3PO4
Pyrophosphoric acid is a medium-strength inorganic acid.
Pyrophosphoric Acid, H4P2O7:
Diphosphate is an acyclic phosphate anhydride obtained by condensing two molecules of phosphoric acid. It plays a role as a metabolite of E. coli. Phosphorus pentoxide and acyclic phosphate anhydride. It is a conjugate acid of diphosphate (1). Pyrophosphate is a metabolite contained in Escherichia coli (K12 strain, MG1655).
Pyrophosphate is a component of radiopharmaceuticals used to visualize bone and cardiovascular abnormalities and is also used as a component of some iron deficiency anemia prevention products.
Formula and Structure: Pyrophosphate is a compound that has a conjugated pyrophosphate base and a conjugated diphosphate. Diphosphate is another name for a chemical substance. The chemical formula of pyrophosphate is H4O7P2.
The chemical formula indicates that hydrogen, oxygen, and phosphate are one of the components involved in the production of compounds. The condensation of two phosphate molecules produces a chemical. The IUPAC name for the compound is Phosphono Dihydrogen Phosphate. The structural formula of diphosphate is as follows.
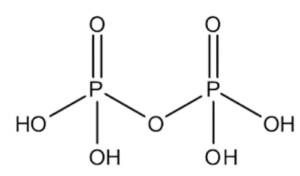
Physical Properties:
The physical properties of a compound can be defined as properties that can be quantified (calculated or visualized) without changing the chemical composition or formula of the compound. Conductivity, physical appearance, melting point, boiling point, and density are all physical properties of a compound.
The following are some of the physical properties of pyrophosphate.
- The physical state of a compound is defined as a solid.
- The melting point of pyrophosphate is 71.5 degrees Celsius.
- Diethyl ether and ethyl alcohol are also soluble in pyrophosphate.
- The polymorph is formed when it crystallizes.
- It is very soluble in cold water and slowly produces phosphoric acid.
- Decomposes much faster with hot water. Soluble in alcohol and ether.
Chemical Properties:
The chemical properties of a compound can be defined as the properties of a substance that are quantified or observed during a chemical reaction that alters the properties or composition of the molecule. Reactivity, toxicity, coordination, flammability, production enthalpy, heat of combustion, oxidation state, and chemical stability with other chemicals are all chemical properties.
Hazards and Dangers:
- Accidental eating of the substance can be fatal. According to animal studies, ingestion of substances weighing less than 150 grams can be fatal or cause serious health problems.
- This substance can cause burns to the oral cavity and gastrointestinal tract after ingestion.
- Acid Caustic Alkali can cause burns to the mouth, throat and esophagus when ingested. Direct contact with the
- Material may cause eye burns. Vapors and mists can cause serious eye injuries.
- Contact with corrosive acids on the skin can cause discomfort and burns. They are severe at clear boundaries and can heal slowly with the formation of scar tissue.
- This substance can cause respiratory distress in some people. The body’s reaction to discomfort can exacerbate lung damage.
Uses:
- Catalysts for the production of organic phosphate esters, metal treatments and stabilizers for organic peroxides.
- Vanadyl pyrophosphate methoxy cluster anion can be prepared with pyrophosphate. Due to its ability to form complexes, this acid is used to bind the anions of inorganic phosphates.
- Pyrophosphate is a radiopharmaceutical used to study bone and cardiovascular abnormalities. It is also found in some medicines that help prevent iron deficiency anemia.
Conclusion:
Pyrophosphate is the conjugate base of Pyrophosphoric Acid and is corrosive in addition to poisonous in nature. Pyrophosphoric Acid is a medium-sturdy inorganic hygroscope through nature and is colorless in addition to odorless chemicals. Anions, salts, and esters of pyrophosphoric acid are referred to as pyrophosphates. Let us realize the chemical homes of Pyrophosphoric Acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




