Aromatic hydrocarbons are organic molecules with a circular structure and sigma bonds as well as delocalized pi electrons. Arenes or aryl hydrocarbons are other names for them.
“Unsaturated hydrocarbons with one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are linked” are aromatic hydrocarbons. A benzene ring can be found in several aromatic hydrocarbons (also referred to as an aromatic ring). The benzene ring is stabilised by resonance, and the pi electrons in the ring structure are delocalized.
Following are a few examples of aromatic hydrocarbons. All of these compounds have a benzene ring, as can be seen.
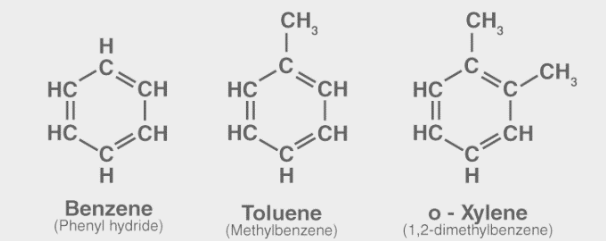
Look into Xylene.
Heteroarenes are aromatic hydrocarbons that do not have a benzene ring. Huckel’s rule (total number of pi electrons in a monocyclic ring = 4n + 2 where n is any positive integer or zero) applies to all of these heteroarenes.
A minimum of one carbon is replaced by either nitrogen, oxygen, or sulphur in these compounds. Furan (which contains oxygen) and pyridine are two examples of heteroarenes (contains nitrogen).
Aromatic Hydrocarbon Properties
“Benzene was the first compound to be classified as an aromatic hydrocarbon.” It’s also the most difficult aryl hydrocarbon to understand. Each carbon atom in the benzene ring has two carbon-carbon sigma bonds, one carbon-hydrogen sigma bond, and one delocalized pi electron double bond with a neighbouring carbon.
A circle inside the hexagon represents the delocalization of pi electrons in the benzene molecule. The bond order of all carbon-carbon bonds in this molecule is assumed to be 1.5, and this equivalency may be explained using benzene resonance structures.
The following are some general characteristics of aromatic hydrocarbons.
- Aromaticity is present in these substances (additional stability granted by resonance)
- In these types of compounds, the carbon to hydrogen atom ratio is relatively high.
- When burned, aromatic hydrocarbons produce a yellow flame that is intense and sooty.
- Electrophilic substitutions and nucleophilic aromatic substitution processes are common in these molecules.
- These compounds might be monocyclic or polycyclic, it should be emphasised.
Aromatic Hydrocarbon Reactions
Aromatic hydrocarbons are used as the main reactant in many organic chemical processes. This subsection includes a list of such reactions as well as a brief description of each one.
Reactions of Aromatic Substitution
One substituent on the ring of an aromatic hydrocarbon, usually a hydrogen atom, is replaced by a different substituent group in these processes.
The following are examples of aromatic substitution reactions:
- Aromatic nucleophilic substitution reactions
- Aromatic electrophilic substitution reactions
- Aromatic radical nucleophilic substitution reactions
- The electrophilic substitution seen in the nitration reaction of salicylic acid is an example of an aromatic substitution reaction.
Reactions That Couple
The coupling of two radical fragments is done with the help of a metal catalyst in these reactions. The following types of bonds can form when aromatic hydrocarbons undergo coupling processes.
The coupling processes of arenes can produce carbon-carbon bonds, resulting in compounds such as vinyl arenes, alkyl arenes, and so on.
In these processes, carbon-oxygen bonds can form, resulting in aryloxy molecules.
In coupling reactions, carbon-nitrogen bonds can form, resulting in compounds like aniline.
The arylation of perfluorobenzene, as shown below, is an example of a coupling reaction using aromatic hydrocarbons.
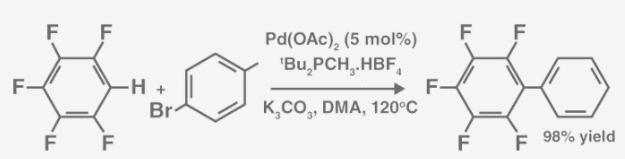
Palladium (II) acetate is the catalyst in this process. It’s also worth noting that DMA stands for Dimethylacetamide.
Reactions of Hydrogenation
Arenes hydrogenation processes usually result in the creation of saturated rings. The reduction of 1-naphthol into a variety of various isomers of decalin-ol is an example of such a process.
The hydrogenation of resorcinol with the help of spongy nickel (also known as Raney nickel) and aqueous NaOH is another example of such a process. This process starts with the production of an enolate, which is then alkylated (with methyl iodide) to produce 2-methyl-1,3-cyclohexanedione.
Aromatic Hydrocarbon Applications
Aromatic hydrocarbons are widely used in biological and synthetic processes. The following are some of the many applications for aromatic hydrocarbons.
Chlorophyll, the green pigment found in plants, is made up of aromatic hydrocarbons and plays a crucial role in the generation of food in plants.
These aromatic hydrocarbons are also found in nucleic acids and amino acids in the human body.
In model glues, methylbenzene, an aromatic hydrocarbon, is employed as a solvent.
The chemical naphthalene is used in the manufacture of mothballs.
Phenanthrene is an aryl hydrocarbon used in the production of medicines, dyes, and explosives.
TNT, or trinitrotoluene, is an important aromatic hydrocarbon that is commonly employed in explosives.
Aromatic hydrocarbons are widely used in the plastic and petrochemical industries.
Aromatic Polycyclic Hydrocarbons
These are hydrocarbons that are made up of fused aromatic rings. These can be present in coal, tar, oil, and some prepared meals like smoked salmon and burnt toast, among other things.
Naphthalene is a common example of these polycyclic hydrocarbons. These substances are considered contaminants.
Methylbenzene, Naphthalene, Phenanthrene, Trinitrotoluene, and o-dihydroxybenzene are examples of aromatic hydrocarbons.
Conclusion
We can conclude that unsaturated and aromatic hydrocarbons are only marginally soluble in water; toluene’s solubility in water is less than 500 mg/L. Cyclohexene has a lower concentration. In water, cyclohexane is entirely insoluble. In these types of compounds, the carbon to hydrogen atom ratio is relatively high. When burned, aromatic hydrocarbons produce a yellow flame that is intense and sooty. Electrophilic substitutions and nucleophilic aromatic substitution processes are common in these molecules.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




