An amine is an organic compound and is considered a derivative of ammonia in general. We can replace one, two, or all three hydrogen atoms of an ammonia molecule with alkyl and aryl groups to produce these nitrogen-based organic compounds,
We prepare amines by the following methods:
Reduction of nitro compounds
Nitro compounds are capable of getting reduced to corresponding amines. It happens when hydrogen gas is passed over them in the presence of nickel, palladium, or platinum. Nitro compounds can also reduce metals to produce amines.
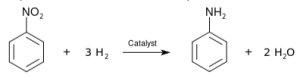
The reaction happens when aliphatic nitro compounds are reduced to their corresponding alkanamines. It is common to minimize nitro compounds with iron scrap and hydrochloric acid. The FeCl2 produced during the reaction is hydrolysed and released as hydrochloric acid. A small amount of Hydrochloric acid is required to kick start the reaction.
Ammonolysis of alkyl halides
A nucleophilic substitution reaction happens when a benzyl halide or alkyl reacts with ethanolic ammonia solution. Due to the reaction, the amino (–NH2) group replaces the halogen atom in the halide.
NH3 + RX → RNH3X
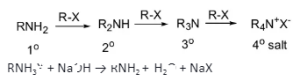
RNH3X + NaOH → RNH2 + H2O + NaX
Ammonolysis occurs in a sealed tube at 373K temperature. The nucleophile of the primary amine obtained from the reaction keeps reacting with the alkyl halide. The reaction doesn’t stop until alkyl groups replace all the hydrogen atoms of amine. As a result of this reaction, secondary and tertiary amines are produced along with a quaternary ammonium salt.
Ammonolysis also creates a quaternary ammonium salt and a primary, secondary, and tertiary amine mixture. Ammonia, on the other hand, produces primary amine. Halides react with amines in the following order: RI>RBr>RCI.
Reduction of nitriles
Using lithium aluminum hydride to reduce nitriles creates primary amines (LiAIH4) or Catalytic hydrogenation of nitriles produces primary amines. This reaction produces amines with one more carbon atom than the original amines ascending in the anime series.
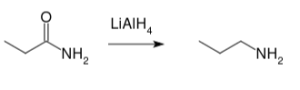
![]() Reduction of amides
Reduction of amides
Lithium aluminum hydride reduces amines to their corresponding amides. Substituting NN and N, N, NN amides produces secondary amines.
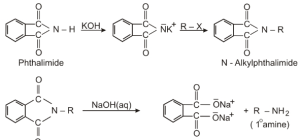
Gabriel phthalimide synthesis
We only use Gabriel phthalimide synthesis to produce primary amines using. By treating phthalimide with ethanolic potassium hydroxide, we may make the potassium salt. We can produce the primary amine by heating this potassium salt with an alkyl halide and alkaline hydrolysis. This synthesis method only produces primary aliphatic amines and not primary aromatic amines because the nucleophilic replacement of aryl halides by the phthalimide anion does not occur.
Hoffmann bromamide degradation reaction
It is possible to convert amines directly into their amides. We treat amide with a KOH+Br2 solution containing a base and bromine (KOH+Br2). We treat an amide with bromine in an ethanolic or aqueous sodium hydroxide solution to produce primary amines. The alkyl or aryl group migrates from the carbonyl carbon of the amide group to the nitrogen atom in this degradation reaction. The amine, thereby formed, has one less carbon than the amide group.
RCONH2 + Br2 + NaOH → RNH2 + Na2CO3 + 2NaBr + H2O
Amide Amine
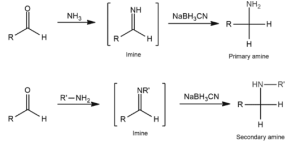
Reductive amination of aldehydes and ketones
Catalytic reduction of carbonyl compounds such as aldehydes or ketones in ammonia results in the formation of primary, secondary, or tertiary amines.
After catalytic reduction with sodium cyanoborohydride, the reaction of an aldehyde or ketone with ammonia results in the formation of an amine.
After reduction by sodium cyanoborohydride,aldehyde or ketone react with primary amines to form NNN-substituted amines.
After aldehyde or ketone reacts with secondary amines, catalytic reduction by sodium cyanoborohydride results in N, NN, and N-substituted amines.
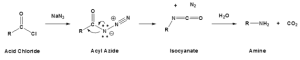
Curtius reaction
We can also prepare amines by reacting acid chloride with sodium azides, forming isocyanate, then hydrolysed to amines.
Schmidt reaction
In Schmidt’s reaction, we can make amines with azide and carboxylic acid.
Conclusion
Substituting one or more hydrogen atoms in ammonia (NH3) molecules with an alkyl or aryl group produces organic compounds known as amines. Proteins, vitamins, alkaloids, and hormones all contain amines. Polymers, dyes, and pharmaceuticals are examples of synthetic materials and adrenaline and ephedrine, two biologically active compounds, have a secondary amino group, raising blood pressure. Antihistaminic drug Benadryl also contains a tertiary amino group. We use surfactants made from quaternary ammonium salts. We use Diazonium salts as intermediates to produce various aromatic compounds, including dyes. We can create Amines by replacing one, two, or three hydrogen atoms with alkyl groups.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





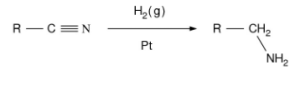 Reduction of amides
Reduction of amides