Alcohols are molecules that include one or more hydroxyl (-OH) groups that are directly connected to a carbon chain, as opposed to other compounds. Generally speaking, alcohols in the free-form are not found in nature; instead, they are found mostly in the essential or volatile oils extracted from the flowers, leaves, and stems of various plants.
Preparation of Alcohols
1. Hydrolysis of Halides
Alcohol is formed when alkyl halides are heated in the presence of an aqueous solution of an alkali hydroxide through a nucleophilic substitution process.
R-X + KOH → R-OH + KX
The primary and secondary alcohols are produced by this general method. Glycerol can be generated from propylene through a number of processes, one of which being the hydrolysis of a halide, as part of a multi-step procedure.
2. Hydration of Alkenes
The addition of water in the presence of a catalyst causes direct hydration to take place.
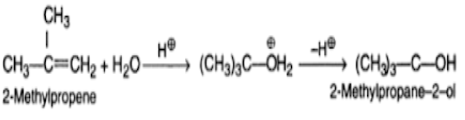
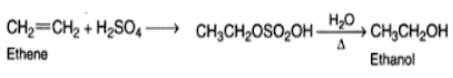
Indirect hydration is performed by adding sulphuric acid to an alkane and then hydrolyzing the alkyl hydrogen sulphate formed as a result of the reaction.
3. Hydroformylation of Alkenes
In the presence of a catalyst, lower molecular weight olefins react with carbon monoxide and hydrogen to generate hydroformylation, also known as the oxo reaction, which is a chemical process.
The resultant aldehyde is next hydrogenated in order to generate an alcoholic compound.
4. Alkene Hydroboration by Hydrogen Peroxide
When alkenes are treated with diborane, alkyl boranes (R3B) are formed. When alkylboranes are oxidised with alkaline hydrogen peroxide, alcohol is produced.
Hydroboration of Alkenes
5. Carbonyl Compounds are reduced in concentration.
The reduction of aldehydes and ketones can also result in the formation of alcohols. Aldehydes can be converted to primary alcohols, and ketones can be converted to secondary alcohols. This procedure can be accomplished by the use of catalytic hydrogenation or chemical reducing agents such as lithium aluminium hydride, LiAlH4.
Such reduction processes play a crucial role in the preparation of some alcohols, which are less readily available than the corresponding carbonyl compounds in the environment. Sodium borohydride, or NaBH4, is important to notice because it does not dissolve or break down carbon-carbon double bonds, even when they are conjugated with carbonyl groups.
6. Reduction of Acids to Alcohols
Lithium aluminium hydride (LiAlH4) is one of the few reagents that may convert an acid into an alcohol. LiAlH4 is used in the production of lithium aluminium hydride.
The reaction 4RCOOH + 3LiAlH4 produces 4RCH2OH and 1oalcohol.
A typical ingredient in the laboratory for the reduction of acids, as well as many other kinds of chemicals, LiAlH4 is popular due to the great yields it provides.
7. Other Methods of Preparation of Alcohols
Primary Amines are affected by the action of Nitrous Acid.
When R-NH2 and HNO2 react, R-OH and N2 are formed, and water is formed.
However, under similar conditions, CH3NH2 gives CH3-O-N=O or CH3OCH3
CH3NH2 + 2HNO2 → CH3-O-N=O + 2H2O + N2
Preparation of Alcohols from Grignard Reagent
With the help of Grignard reagents and carbonyl compounds, we can synthesise the three different forms of monohydric alcohols (primary, secondary, and tertiary alcohols). The addition of RMgX to carbonyl compounds, together with hydrolysis, results in the formation of alcohols. The Grignard reagent is a type of organometallic compound in its most basic form. Let’s take a closer look at this reaction because it is really crucial to understand.
In the presence of metallic magnesium overturnings, we observe an explosive reaction between a solution of alkyl halide in dry ethyl ether, (C2H5)O, and the alkyl chloride. We can see that the solution has turned cloudy and is starting to boil up. The magnesium metal is gradually depleted of its properties. The Grignard reagent is the solution that results as a result of this reaction.
CH3I + Mg → CH3MgI
H3CH2Br + Mg → CH3CH2MgBr
Ethyl bromide is a chemical compound that can be used to make a variety of products. Ethylmagnesium bromide is a chemical compound.
The Grignard reagent is represented by the generic formula RMgX and is referred to as alkyl magnesium halide in general.
Grignard Synthesis of Alcohols
The type of alcohol that we acquire from a Grignard synthesis is determined by the type of carbonyl compound that we utilise in the reaction: formaldehyde, HCHO, yields primary alcohols, whereas acetic acid yields secondary alcohols. Aldehydes, on the other hand, give rise to secondary alcohols, while ketones, R2CO, give rise to tertiary alcohols.
What is the best way to obtain this? It is straightforward. Formaldehyde, higher aldehyde, and ketone are all carbonyl compounds that are distinguished by the number of hydrogens linked to the carbonyl carbon. The carbonyl carbon is the one that is responsible for the final formation of the –OH group in the product; the number of hydrogens in the alcohol determines whether it is primary, secondary, or tertiary.
OR 2CH3NH2 + 2HNO2 → CH3OCH3 + 2N2 + 3H2O
Conclusion
The most common reactions involving alcohols can be divided into five categories: oxidation, dehydration, substitution, esterification, and reactions involving alkoxides.Oxidation is the most common reaction involving alcohols, followed by dehydration and substitution.
Alcohols are among the most frequent organic molecules found in the environment. In addition to being employed as sweeteners and scent ingredients, they are also valuable intermediates in the synthesis of other compounds, and they are among the most widely produced organic chemicals in the industrial sector.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






