A chemical addition reaction, also known as a Markownikoff reaction, can be described using Markovnikov’s Rule, which is sometimes known as Markownikoff’s rule. When this rule was initially proposed in 1865, it was by the Russian chemist Vladimir Vasilyevich Markovnikov.
What is Markovnikov’s Rule, exactly?
Addition of a protic acid to an asymmetric alkene results in the acidic hydrogen attaching itself to the carbon atom with the greatest number of hydrogen substituents and the halide group attaching itself to the carbon atom with the greatest number of alkyl substituents.
To make the rule even more straightforward, it might be phrased as follows: “Hydrogen is added to the carbon containing the greatest number of hydrogens, and the halide is added to the carbon containing the least number of hydrogens.”
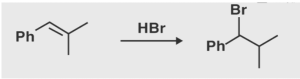
An example of a reaction that follows Markovnikov’s rule is the addition of hydrobromic acid (HBr) to propene, which is depicted in the illustration below.
Markovnikov’s rule
According to the reaction described above, the vast majority of the products generated follow Markovnikov’s rule, but only a small fraction of the products fail to do so.
Consider the addition reaction, which occurs when an alkene combines with water to produce an alcohol as a result of the reaction. The production of a carbocation is the mechanism by which this reaction takes place. In this reaction, it is found that the hydroxyl group attaches itself to the carbon with more carbon-carbon bonds, but the hydrogen atom attaches itself to the other carbon in the double bond, which has more carbon-hydrogen bonds, as observed in the previous reaction.
Why does Markovnikov’s Rule work? What is the mechanism that makes it work?
Consider the same example as before, namely the addition reaction of hydrobromic acid with propene, in order to better comprehend this mechanism. In order to understand how Markovnikov’s rule works, it is necessary to break it down into two steps.
First, we’ll have a look at what to do.
The alkene is protonated, and this results in the formation of the more stable carbocation, as indicated in the diagram below.
Step 1 of the Markovnikov Rule Mechanism
The image above shows that there are two types of carbocations that can be created from the protonation of an alkene: a primary carbocation and a secondary carbocation. The main carbocation is the most common type of carbocation, and the secondary carbocation is the least common type. The secondary carbocation, on the other hand, is significantly more stable, and as a result, its production is preferred over the formation of a primary carbocation.
Step 2 of the Markovnikov Rule Mechanism
The carbocation is now being attacked by the halide ion nucleophile. The alkyl halide is formed as a result of this reaction. The creation of the secondary carbocation is favoured in this reaction; hence, the primary product would be 2-bromopropane, as represented in the diagram below.
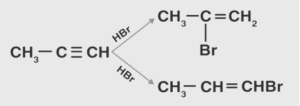
It is crucial to note that the Markovnikov’s rule was designed exclusively for use in the addition reaction of hydrogen halides to alkenes, and that it was not intended for use in any other reaction. Anti-Markovnikov addition reactions are those that are the polar opposite of ‘Markovnikov’ addition reactions. This is predicated on the fact that the reaction is regioselective.
Addition reactions involving Markovnikov and Anti-Markovnikov are illustrated in the following examples.
The Hydration of Alkenes is a process that occurs in the presence of water.
When certain aqueous acids (often sulfuric acid) are used to treat alkenes, the resultant electrophilic addition process produces an alcohol as the end product. Markownikoff’s rule can be used to forecast the regioselectivity of such reactions in advance. This classification is applicable to all Markovnikov reactions, as a result of the foregoing. As an electrophile, the H+ ion attacks the alkene, resulting in the formation of a carbocation intermediate in the process of hydration of alkenes (the intermediate with greater stability is protonated). The following nucleophilic attack on the carbocation by water molecules results in the formation of an oxonium ion, which is then deprotonated to yield the desired alcohol product as a byproduct.
Alkenes are subjected to hydroboration and oxidation.
When alkenes are treated with borane (BH3) in the presence of hydrogen peroxide or sodium hydroxide, the resultant product is an alcohol, which is the ultimate product. In this electrophilic addition process, the boron atom functions as an electrophile, and the other elements participate as electrophiles. It is possible to classify this reaction as an anti-Markovnikov reaction because it does not follow Markovnikov’s rule and hence does not follow his rule.
Conclusion
It was discovered primarily for the addition of hydrogen halides to alkenes.
Unsymmetrical alkenes obey Markovnikov’s rule.
Markovnikov’s rule governs electrophilic addition reactions of aromatic alkenes and alkynes.
The main product is stable carbocation.
The protonation step determines the rate.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out