Fractional distillation is a type of distillation that involves the separation of miscible liquids into two or more fractions. Multiple distillations and condensations are carried out repeatedly, and the resulting mixture is usually separated into its constituent parts. The separation occurs when the mixture is heated to a temperature at which fractions of the mixture begin to vaporize, which is determined by the manufacturer.
The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures, which is why it is called differential distillation. In this case, when the mixture is heated, the substance with the lower boiling point begins to boil and condense first, eventually converting to vapour.
Procedural for Fractional Distillation
It only takes a few fractional distillation apparatuses to complete the process. It is made up of a distilling flask, a condenser, a receiver, a fractionating column, a thermometer, and a heat source, among other things.

Following the setup of the apparatus, a mixture of two miscible liquids A and B is prepared, with A having greater volatility than substance B in this case. A portion of the solution is poured into the distilling flask while the fractionating column is connected to the flask’s tip with a rubber band. Heat is applied, resulting in a gradual increase in temperature. The mixture then begins to boil, and vapors begin to rise to the top of the flask. The volatile component A is responsible for the vapors. The vapors then begin to move through the fractionating column and into the condenser, where they are cooled and condensed to form a liquid, which is collected in the receiver after passing through the receiver.
In the course of the process, vaporization and condensation occur repeatedly until the two mixtures are completely separated.
Distillation in the Industrial Sector
A popular separation technique in a variety of industries, fractional distillation is one of the most widely used separation techniques. While the underlying principle of the process remains the same, the distillation is carried out on a larger scale than in the previous version. Distillation columns, also known as distillation or fractionation towers, are massive vertical cylindrical columns that are used in the distillation process. Industrial towers employ reflux to ensure complete separation of the various mixtures being processed.
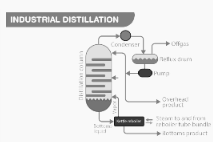
Fractional Distillation
Fractional Distillation of Crude Oil is a method of distilling crude oil into smaller fractions.
The separation of different components of crude oil is a common application of fractional distillation in the industrial setting. Crude oil typically contains a mixture of substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil, and kerosene, among other things. The distillation process aids in the effective separation of these components from one another.
High-pressure steam is used to heat the crude oil that has been added to the chamber. The mixture begins to boil, resulting in the formation of vapor. This is the point at which a variety of substances transition into the vapor phase. After passing through the fractional distillation column, which is composed of several plates, the vapor rises to the surface. Each of the plates has holes in it, which allows the vapor to pass through them. The temperature at the top of the fractionating column is typically kept at a low level. Components with the highest boiling points will condense in the lower part of the column, whereas substances with a low boiling point will condense at the top of the column in this case. In the following step, the condensed vapors or liquid fractions are drawn away from the columns’ sides. To cool them even further, the liquid fractions collected can be passed through condensers after being collected.
Fractional Distillation Has a Variety of Applications
- A technique known as fractional distillation is used for water purification as well as the separation of acetone and water.
- Fractional distillation is used in a variety of industries, including oil refineries and chemical plants, to purify and separate a wide range of organic compounds, with the primary goal of purification and separation.
- The separation of (liquefied) air is also accomplished through the use of fractional distillation. It is possible to obtain components such as liquid nitrogen and oxygen, as well as concentrated argon.
- Distillation is used in the production of high-purity silicon from chlorosilanes, which is a byproduct of the process. Silicon is a material that is widely used in semiconductors.
Conclusion
Fractional distillation is a type of distillation that involves the separation of miscible liquids into two or more fractions. Multiple distillations and condensations are carried out repeatedly, and the resulting mixture is usually separated into its constituent parts.A popular separation technique in a variety of industries, fractional distillation is one of the most widely used separation techniques. While the underlying principle of the process remains the same, the distillation is carried out on a larger scale than in the previous version.Fractional Distillation of Crude Oil is a method of distilling crude oil into smaller fractions.
The separation of different components of crude oil is a common application of fractional distillation in the industrial setting.Distillation is used in the production of high-purity silicon from chlorosilanes, which is a byproduct of the process. Silicon is a material that is widely used in semiconductors.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




