A chemical process that converts primary alkyl halides to primary amines is known as the Gabriel synthesis. Traditionally, potassium phthalimide is used in the reaction. Siegmund Gabriel, a German chemist, inspired the reaction’s name.
Gaabriel Synthesis
By adopting the Gabriel synthesis, you may avoid excessive alkylation, which is the most significant advantage. The reaction of potassium hydroxide with phthalimide also results in the formation of an excellent nucleophile in the form of an imide ion, which is useful in organic synthesis. The imide ion undergoes a nucleophilic substitution reaction with the alkyl halide, resulting in the formation of an intermediate compound called N-alkyl phthalimide. The hydrolysis or hydrazinolysis of this phthalimide results in the formation of a primary alkyl amine. Aryl amines, on the other hand, cannot be synthesised using the Gabriel method because aryl halides do not undergo simple nucleophilic substitution.
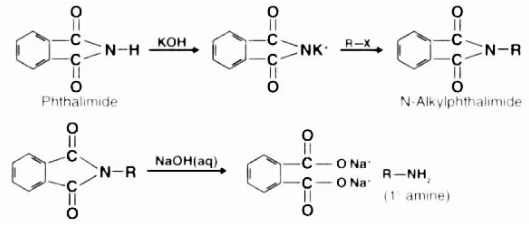
Gabriel Phthalimide Synthesis Mechanism
Step 1
As a result of the introduction of potassium hydroxide into the phthalimide, an acid-base reaction occurs. The deprotonation of the imide is caused by the hydroxide ion. This results in a proton that is more acidic than any simple amine (due to the presence of two neighbouring carbonyl-like groups that provide resonance stabilisation), culminating in the formation of a powerful nucleophile, the imide ion.
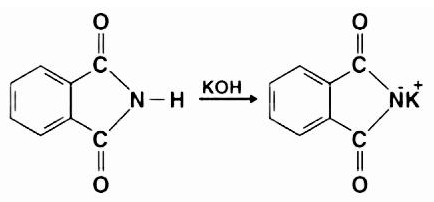
Step 2
It is the nucleophilic imide ion that hits the electrophilic carbon of the alkyl halide that causes the reaction. The nitrogen atom then takes the place of the halogen (Fluorine, Chlorine, Bromine, or Iodine) in the alkyl halide and forms a connection with the carbon atom. It is this reaction that results in the creation of an N-Alkyl Phthalimide.
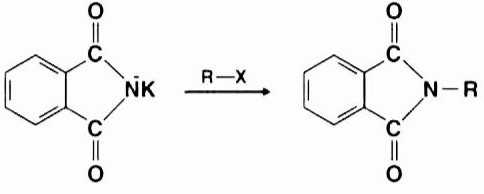
Step 3
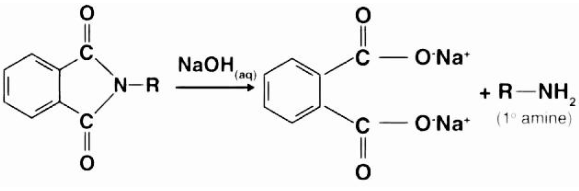
The mechanism that takes place here is quite similar to that of base-catalyzed hydrolysis of esters, with the exception that nitrogen is linked to the R group instead of oxygen in this case. Cleavage of N-Alkyl Phthalimide occurs when the hydroxide ion assaults a carbon-nitrogen bond that has formed between them. This attachment occurs when the cation in the base binds itself to the oxygen atom as well. It is vital to remember that when the oxygen atom replaces the nitrogen atom in the phthalimide, the nitrogen atom linked to the R group forms a bond with the hydrogens expelled from the hydroxide ion. The following illustration depicts an example of the third stage in the Gabriel phthalimide production procedure.
Alternative Gabriel Reagents
To supplement the usage of phthalimides, many other reagents have been created. Most of these reagents (for example, the sodium salt of saccharin and di-tert-butyl-iminodicarboxylate) are electrically comparable to phthalimide salts, which are composed of imido nucleophiles. These reagents have the benefit of hydrolyzing more easily, extending the reactivity to secondary alkyl halides, and allowing the formation of secondary amines.
Conclusion
To summarise, the Gabriel method can be used to obtain primary amines from phthalimides, which is a useful technique. In addition to utilising an aqueous base, acidic hydrolysis or hydrazinolysis can be used to follow the mechanism instead of using an acidic base (as was shown in the mechanism above). As a general rule, the Gabriel technique is ineffective with secondary alkyl halides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




