The complete transfer of valence electrons between atoms is known as ionic bonding. It’s a type of chemical bond that results in the formation of two ions with opposite charges. In ionic bonding, the metal loses electrons to become a positively charged cation, while the nonmetal gains them to form a negatively charged anion. The metal loses electrons to be a positively charged cation in ionic bonding, until the nonmetal receives those electrons to become a negatively charged anion. Ionic bonds require an electron source (typically a metal) and an electron acceptor usually a nonmetal.
Because metals have few electrons in their outermost orbitals, ionic bonding occurs. By shedding those electrons, these metals can achieve noble gas configuration and meet the octet rule. Nonmetals having valence shells containing close to 8 electrons, on the other hand, are more likely to receive electrons to produce a noble gas structure.
To satisfy the octet state in ionic bonding, more than one electron can be donated or received. The charges on the anion and cation correspond to the number of electrons delivered or received. In ionic bonding, the compound’s net charge should be zero.
Definition
“Ionic bonding happens when electrons are transferred between atoms and ions are formed in the process. The heart of the ionic bond is the electrostatic attraction between newly created cations and anions.”
There are numerous terminologies used in the Ionic Bonding, which are given here.
One atom
An atom is a single-celled piece of matter that defines a chemical element. A nucleus in the centre of an atom is normally surrounded by one or more electrons. Every electron has a negative charge attached to it. The nucleus is positively charged and comprises one or more heavy protons and neutrons.
Cation
A cation is a positively charged ion, meaning it has more protons positively charged particles than electrons negatively-charged particles. When an atom loses one or more electrons, a cation is formed: the loss of the negatively charged electron(s) results in a positive overall charge.
Anion
An anion is an atom that has gained electrons and has a negative charge. In reality, whether the superoxide anion is generated by oxidative radicals or by an interaction between a solvated electron and molecular oxygen is yet unknown. The anion has a negative charge, while the cation has a positive charge.
Ionic bonding formation
When two or more atoms lose or gain electrons to create an ion, an ionic bond can be formed. Metals that lose electrons form ionic links with nonmetals that gain them. Ions with opposite charges attract each other and form an ionic connection. Atoms are bound in an ionic bond by the attraction of opposing ions, whereas atoms are bound in a covalent bond by sharing electrons.
Example of Ionic Bonding
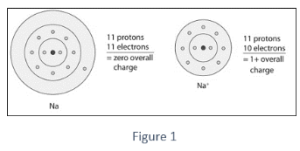
To reach octet configuration, this sodium molecule contributes the lone electron in its valence orbital. Due to the loss of an electron, a positively charged cation is formed.
Ionic Bonding properties
The properties of ionic bonding are given below.
- Ionic compounds are formed when atoms make ionic bonds with one another.
- The strongest sort of chemical link is an ionic bond, which results in unique features.
- The bond has a partial positive charge on one atom and a partial negative charge on the other.
- Polar chemicals, on the other hand, frequently dissolve in water. Ionic chemicals are therefore good electrolytes.
- Ionic bonded chemicals have very high melting and boiling points.
- In their aqueous solutions or molten state, ionic bonded molecules are excellent conductors of electricity. This is because ions, which act as charge carriers, are present.
Conclusion
In this article we learned, the term “ionic bond” refers to a chemical bond that produces two oppositely charged ions. The entire transfer of valence electrons is referred to as this bonding. Ionic bonds are significant because they allow certain chemical molecules to be synthesized. Ionic characteristics and interactions can be manipulated by scientists to create desired results. Because most carbon compounds interact predominantly through covalent bonding, covalent bonds are particularly significant.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




