We discovered that describing the bond angles and lengths of methane allowed us to define its entire three-dimensional form. Ethane, which is made up of two methyl groups joined together, exhibits properties that are extremely similar to methane. However, because ethane can rotate internally around its C-C bond, the whole 3-dimensional structure of the gas cannot be determined only by these bond lengths and angles.
Consider the cylindrically symmetric character of sigma bonds to understand why ethane has this extra degree of freedom. While its two ends spin, the sigma bond can maintain a full degree of overlap. As a result, rotation around sigma bonds has a low energy barrier. The C-C sigma bond, unlike pi bonds in alkenes, does not keep the two methyl groups in fixed locations relative to one another. Conformations or conformers are the many spatial arrangements created by rotations around a single link.
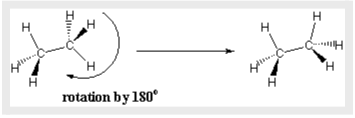
Conformation Visualisation
Organic chemists utilise a variety of techniques to visualise the conformations of molecules. Wedges imply bonds that stretch out from the plane of the page toward the reader, whereas Dashes denote bonds that go into the plane of the page away from the reader in one of these techniques. The tetrahedral geometry of sp3-hybridised carbons is usually represented using this notation.
A Newman projection can be used to precisely describe the conformation of a specific bond. A Newman projection is a direct view down the bond of interest. The atom in front of the bond is represented by the circle in the Newman projection, and the lines radiating from the centre are the atom’s bonds. The back atom’s bonds protrude from the circle’s sides.
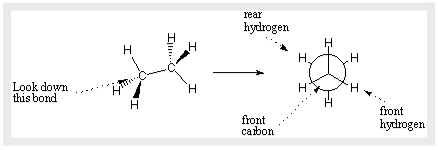
The angles created between bonds on the front atom and bonds on the back atom define Newman projections. Dihedral angles are the name for such angles. Bond lengths, bond angles, and dihedral angles can all be used to define a molecule’s whole 3D form.
Ethane Conformations
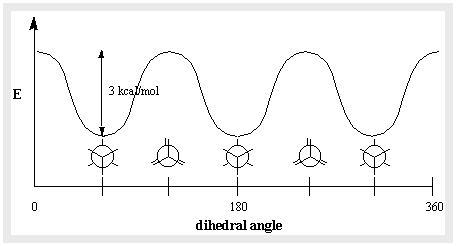
While any sigma bond can have an endless number of conformations, two specific conformers in ethane stand out and have unique names. The C-H bonds on the front and rear carbons are aligned with each other with dihedral angles of 0 degrees in the eclipsed conformation. With dihedral angles of 60 degrees, the C-H bonds on the back carbon lay between those on the front carbon in the staggered conformation.
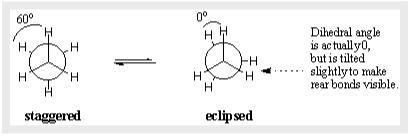
Not all conformations are equally favoured energetically. Ethane’s eclipsed conformation is 3 kcal/mol less stable than the staggered conformation. Because the angles between C-H bonds on the front and back carbons are maximised at 60 degrees, the staggered conformation is the most stable of all potential ethane conformations. The electron concentrations on the C-H bonds are closer together in the eclipsed form than they are in the staggered form. When two C-H bonds are brought together at a dihedral angle of zero degrees, their electron clouds repel each other, raising the molecule’s energy. We can infer that each eclipsed C-H “costs” around 1 kcal/mol since the eclipsed conformation of ethane has three such C-H eclipsing interactions.
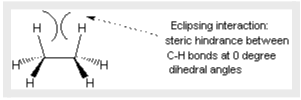
Steric Obstacle
Eclipsing interactions are a type of steric hindrance, which happens when bulky sections of a molecule reject other molecules or other parts of the same molecule. It’s also known as torsional strain since it produces resistance to rotation. Torsional energy is the 3 kcal/mol required to overcome this barrier. It’s worth noting that this value pales in comparison to the 60 kcal/mol necessary to spin around double bonds (the bond energy of a C-C pi bond). Ethane molecules have enough energy to rotate in a steady state at ambient temperature. Because of its fast rotation, it is unable to isolate any specific conformation in the same manner that cis- and trans-alkenes can. Although the phrase “conformational isomer” is sometimes used interchangeably with the term “conformation,” conformations are not real isomers due to their quick interconversion.
Conclusion
As a result, the potential energy associated with various ethane conformations changes with the dihedral angle of the bonds. Despite the fact that the conformers of ethane are in quick equilibrium with one another, the 3 kcal/mol energy differential results in a large preponderance of staggered conformers (> 99.9%) at any one time.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




