Copper (3d10 4s¹) and chromium (3d5 4s¹) electron configurations
This is because the energies of 3d and 4s orbitals are relatively near, and the energy of 3d orbitals decreases as you move across the row.
The arrangement with more electrons in 3d orbitals has a lower energy for both chromium and copper. This is because the difference between 3d and 4s orbital energies is comparable to the pairing energy for chromium (Electron pairs are of higher energy).
The 3d5 4s¹ configuration has the most unpaired electrons of any d-subshell structure, hence it has a lower energy. 3d orbital energy has reduced to the point where 3d orbitals are less energetic than 4s orbitals at copper (at the conclusion of the transition series). However, it would be incorrect to assume that only transition metals may have changing oxidation states. Sulphur, nitrogen, and chlorine, for example, exhibit a wide range of oxidation states in their compounds despite the fact that they aren’t transition metals.
Aside from the transition elements, this variability is less typical in metals. Only lead and tin, two well-known metals from the Periodic Table’s main families, have varying oxidation states.
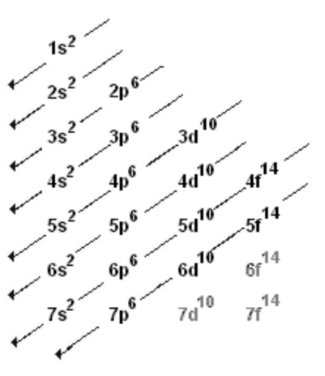
Define Electronic configuration
The chemistry of d-block elements differs significantly from that of their counterparts elsewhere on the periodic table. The composition of their d-electron, which is responsible for some extremely unusual bonding and reactivity, is the fundamental property that distinguishes these metals from others.
We must first grasp what electron configuration is and how it applies before we can discuss d-electron configuration. The arrangement of electrons in metal orbitals is known as electronic configuration.
Every element has a different electronic configuration. It’s crucial to understand the orbitals involved in molecular bonding – s,p,d, and f, which are abbreviations for an element’s coloured, or spectral, lines.
We’re mostly interested in d-orbitals, also known as d-block elements, in transition metals.
Transition metals can have totally or partially filled orbitals, which can modify their oxidation states. Despite the fact that these metals can exist in a variety of oxidation states, they all follow a similar pattern. The number of electrons in the d-orbital rises by one from left to right across the d-block on the periodic table, or from scandium (Sc) to zinc (Zn). Chemists frequently refer to a transition metal’s stable or ground state by its d-label. They might say d-5 to refer to manganese.
What is p orbital?
According to the Heisenberg Uncertainty Principle, an electron can be located at any distance from the nucleus and in any direction at any time. The p orbital is a lobed or dumbbell-shaped area that describes where an electron can be discovered with a high probability. Because the dumbbell’s node is the atomic nucleus, the chances of discovering an electron in the nucleus are extremely slim (but not zero).
The quantum numbers associated with an energy level determine the form of the orbital. Beginning with boron, a three-dimensional tetrahedral structure emerges. It has lost its planarity (2D). In the 2p subshell, there are six protons (B to Ne).
This is the tetrahedron’s third level. The side view of an atomic element is given below, based on the rotation axis. The first four protons (H to Be) have been placed as the tetrahedron’s initial two layers. Neutrons will be removed from view starting with the 2p subshell to make visualising the nucleus structure easier. Neutrons, on the other hand, are thought to continue to fill the spaces between protons.
s and p Block
All elements that have been found so far are included in the periodic table of elements. S block, p block, d block, and f block are the four major groups of these elements. They are classified by the orbital in which their valence electrons are found.
According to their physical properties, these elements can alternatively be classified as metals, non-metals, or metalloids. Except for hydrogen, all s block elements are metals. Non-metals make up the majority of the p blocks elements. Metalloids make up the remainder of the p block. The fundamental distinction between s and p block elements is that the valence electrons of the s block elements are in the s orbital whereas the valence electrons of the p block elements are in the p orbitals.
The elements with their valence electrons in the outermost s orbital are known as S block elements. Because the s orbital can only hold a maximum of two electrons, all s block elements have one or two electrons in their outermost s orbital. The s orbital is always at the conclusion of their electron configuration.
Transition elements have d orbitals filled
Despite being the penultimate shell, the d orbital has a higher energy. They are less steady and more reactive when they have more energy. Therefore, it serves as a valence electron. Thus, the nucleus’s influence on the electrons in the d orbital is minimal. As a result, they are more affected by their environment than the atoms or molecules around them.
Conclusion
This is because the energies of 3d and 4s orbitals are relatively near, and the energy of 3d orbitals decreases as you move across the row. This is because the difference between 3d and 4s orbital energies is comparable to the pairing energy for chromium (Electron pairs are of higher energy). 3d orbital energy has reduced to the point where 3d orbitals are less energetic than 4s orbitals at copper (at the conclusion of the transition series). The number of electrons in the d-orbital rises by one from left to right across the d-block on the periodic table, or from scandium to zinc. Because the dumbbell’s node is the atomic nucleus, the chances of discovering an electron in the nucleus are extremely slim.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




