Dinitrogen trioxide is prepared by mixing equal parts of nitric oxide and nitrogen dioxide. The temperature of NO2 is lowered to 21 degrees Celsius (6 degrees Fahrenheit). Only the liquid and solid states exist for this chemical. It produces a combination of NO and NO2 when heated. Commercially, Dinitrogen trioxide is made by oxidizing NO with air, but it can also be made in the lab by heating a heavy metal’s nitrate, as shown in the equation below.
2Pb(NO3)2 + heat → 2PbO + 4NO2 + O2
Structure And Formation
N–N bonds are usually around the same length as hydrazine bonds (145 pm). However, at 186 pm, the N–N bond in dinitrogen trioxide is extraordinarily lengthy. Dinitrogen tetroxide is one of the nitrogen oxides that has lengthy N–N bonds (175 pm). The Cs symmetry of the N2 O3 molecule makes it planar. Microwave spectroscopy of low-temperature, gaseous N2 O3 provided the dimensions indicated below. The equilibrium favors the formation of constituent gasses.
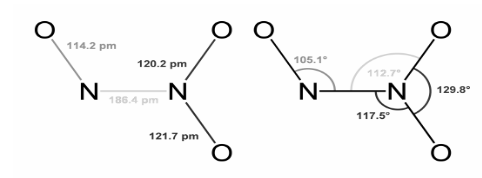
When combined with water, it creates an anhydride of the unstable nitrous acid (HNO2). For the genuine anhydride, a different structure, O=N–O–N=O, may be expected, but this isomer isn’t found. The nitrous acid decomposes into nitric oxide and nitric acid if it is not used up immediately. When N2O3 is added to a base solution, nitrite salts can be produced:
2 NaNO2 + H2O + N2O3 + 2 NaOH
One nitrogen is in the +3 oxidation state here. Dinitrogen trioxide, like other nitrogen oxides, is present in nature as part of the planet’s nitrogen biogeochemical cycle. Nitrogen can be present in the air, oceans, and rivers, as well as soils.
Formula For Dinitrogen trioxide
N2O3 is its chemical formula.
When water and unstable nitrous acid are mixed together, it produces anhydride. HNO2 (nitrous acid) can be broken down into nitric acid and nitric oxide. Mixing N2O3 with base solutions might result in the formation of nitrite salts: The following is an example equation:
N2O3 +2NaOH→2NaNO2+H2O
Nitrogen sesquioxide has a monoisotopic mass of 75.991 grams per mole and an exact mass of 75.991 grams per mole. The total number of hydrogen bond donors is zero, while the total number of hydrogen bond acceptors is four. The number of covalent bound units equals one in this compound, which is canonicalized.
Chemical And Physical Properties of Dinitrogen trioxide
Chemical properties – It’s a common oxidising agent that’s non-flammable, but it can cause flames when coupled with flammable compounds. It generates heat and products like gases when it reacts with reducing agents. The products may be capable of undergoing further reactions, such as combustion in the atmosphere. The ignition of phosphine gas is likewise catalyzed by it. Unless adequately chilled, a mixture of caprolactam dissolved in acetic acid is highly explosive.
Physical properties – It has a deep blue-tinted gas appearance and is easily soluble in water. It has a boiling point of 3.5°C, a melting point of -11.7°C, and a density of 1.4 grams per cubic centimeter.
Production of Dinitrogen trioxide
Dinitrogen trioxide is a deep blue solid with an acidic nature and is formed when NO + NO2 reacts with one other. When mixing of equal quantities of nitric oxide and nitrogen dioxide happens, the mixture is then cooled down below – 21°C to create the chemical compound.
Dinitrogen Trioxide’s Applications
- Special-purpose fuels contain N2O3.
- It is a strong oxidant that can be utilized as an oxidizing agent when coupled with other chemical substances.
- It’s used in the chemical industry to make colors, nylon, and other things.
Hazards of Dinitrogen Trioxide
- N2O3 is a highly hazardous chemical compound.
- If heated, it might blow up.
- If it comes into touch with the skin or is inhaled, it can be lethal, as well as causing severe eye damage and skin burns.
Uses of Dinitrogen Trioxide
- Because of its high combustibility, dinitrogen trioxide is excellent as a special purpose fuel.
- The chemical just aids in combustion and does not burn itself.
- It’s more commonly used in combination with other chemical substances as an oxidizing agent.
Conclusion
Dinitrogen Trioxide is a chemical compound with a deep blue color. It is made by mixing equal amounts nitrogen dioxide and nitric oxide at low temperatures, such as 21 degrees Celsius. It’s a strong oxidant that’s also poisonous and corrosive. Only at low temperatures, i.e. in the liquid and solid phases, is dinitrogen trioxide isolable. It has a vivid blue color in both liquid and solid phases. With Kdiss = 193 kPa (25 °C), the equilibrium favors the constituent gasses at higher
temperatures. The word “nitrogen trioxide” is occasionally used to refer to this chemical, however it actually refers to another compound, the (uncharged) nitrate radical NO3. As part of the planet’s nitrogen biogeochemical cycle, dinitrogen trioxide, like other nitrogen oxides, is found in nature. Nitrogen can be found in the atmosphere, oceans, rivers, and soils.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



