When two or more compounds have the same molecular formula but distinct structural formulas, this is referred to as structural isomerism.
They’re best appreciated in terms of their visual or fully shown structural formula, but we have opted to exhibit them in a number of formats that you’ll need to read, including skeleton formulae.
When isomers are compared, each molecule has at least one atom linked to a distinct atom.
The structural isomerism sub-divisions (a) through (d) are discussed below.
a. Chain isomerism
b. Tautomerism
c. Positional isomerism
d. Functional group isomerism
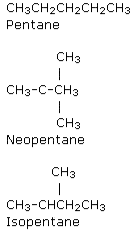
Chain isomers of the alkane molecular formula C5H12 and other shorter/longer alkanes
Note the differences in boiling points between the isomers.
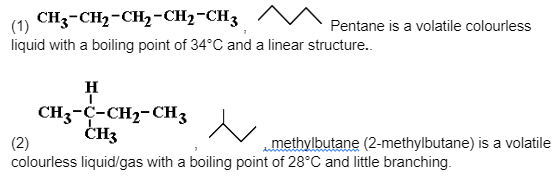
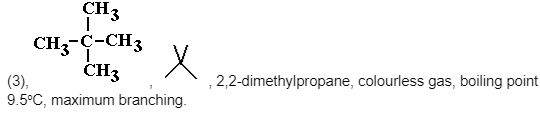
As the molecule grows more branched, it becomes more compact, which is one physical consequence of this isomerism.
As a result of the decreased surface-surface contact, the intermolecular forces (instantaneous dipole-induced dipole forces (weak intermolecular bonding of Van der Waals forces) diminish, and the more compact a molecule is, the more difficult it is to polarise it.
As a result, less and less thermal kinetic energy is required to overcome them, and the boiling point falls from molecule (1) to (3) molecule.Fractional distillation can be used to separate them.
They are chemically highly similar, for example, they all rapidly burn to carbon dioxide and water or react with chlorine and ultraviolet light to generate isomeric halogenoalkanes.
There are no isomers for the lower alkanes CH4, C2H6 or C3H8, but C4H10 has two chain isomers:
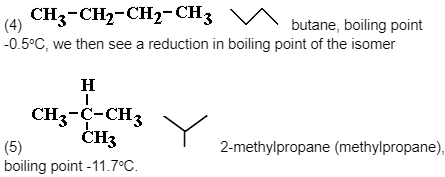
e.g. several of the alkane series’ chain isomers with the chemical formula C8H18
Below are some examples of their structural and skeletal formulas.
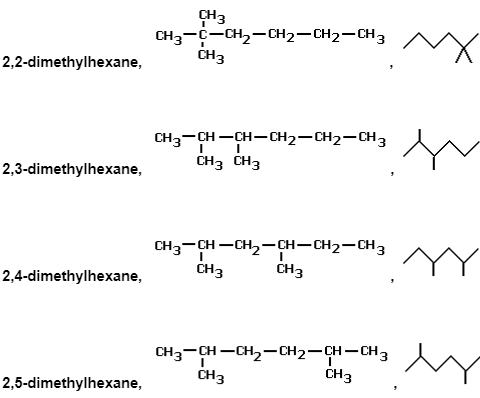
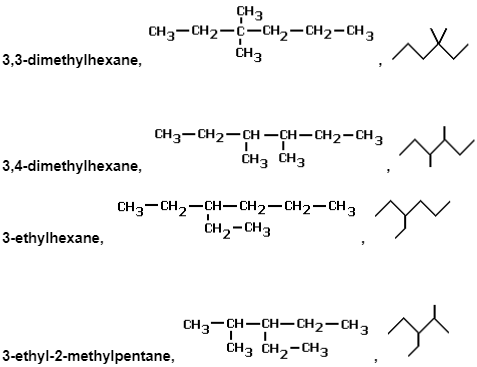
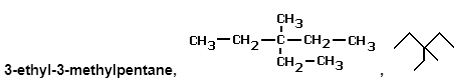
Physically and chemically, they will be very similar molecules, but there will be minor changes in melting point, boiling temperature, and density
The chain isomerisation of alkanes and the octane number of petrol fuels
In the petrochemical industry, chain isomerization is used to manufacture more branched alkanes with a higher octane number from linear alkanes, resulting in fuels that are more appropriate for petrol engines.The amounts of ‘isomers’ in crude oil, as well as the length of the hydrocarbon chain, do not meet specific market demands.Straight chain alkanes are heated with an appropriate catalyst to break up the chains, resulting in the formation of more branched alkanes as well as lower alkanes as the pieces recombine.
The greater the octane number for a given carbon number of an alkane, the more branched the alkane is. The greater the octane number of a fuel/molecule, the less likely it is to produce auto-ignition in an automobile engine, resulting in ‘knocking’ or ‘pinking.’
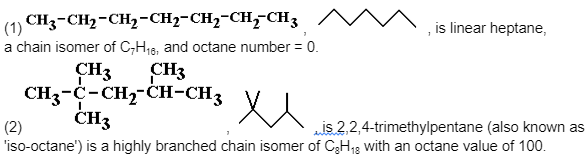
To determine their individual ‘octane rating,’ the known tendency of a combination of (1) and (2) to auto-ignite is compared to that of other fuels/molecules.
One probable isomerisation process of pentane C5H12 is given below using the skeletal formula.
Because they are reversible reactions, altering the reaction circumstances might shift the equilibrium state.
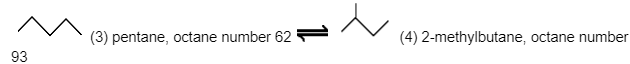
Conclusion
The structural isomers are isomers that differ in the atomic arrangement of molecules without any relation to the spatial arrangement. Structural isomerism is the term for the phenomena of structural isomers.
According to the IUPAC, structural isomerism is also known as constitutional isomerism. In contrast to stereoisomerism, it is a type of isomerism in which molecules with the same chemical formula have various ordering and bondings.
A change in the atomic arrangement of the carbon to the carbon chain of a molecule is known as chain isomerism. Chain isomerism is a feature that occurs when two or more compounds have the same sort of chemical formula but distinct primary chains. Skeletal isomerism is another name for this condition.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out