Phosphorus is required for the survival of all living things. Elemental phosphorus was historically the first element to be separated from human urine, and bone ash was an important early phosphate source, both of which provide evidence of the relationship between phosphorus and terrestrial life. When present in the form of phosphate, it can be found in DNA, RNA, ATP (adenosine triphosphate), and the phospholipids that make up all cell membranes. Low phosphate levels are a significant limiting factor in the growth of some aquatic systems, and the primary commercial application of phosphorus compounds for the production of fertilisers arises from the requirement to replace the phosphorus that plants take from the soil during photosynthesis.
Phosphorus comes in a number of various forms (allotropes), each of which has a distinct set of properties.
White phosphorus and red phosphorus are the two most frequent allotropes of phosphorus.
Scarlet phosphorus is another form of phosphorus that can be created by allowing a solution of white phosphorus in carbon disulfide to evaporate in the presence of sunlight.
Using high pressures and high temperatures, white phosphorus is transformed into black phosphorus (about 12,000 standard atmospheres, or 1.2 gigapascals). Black phosphorus is similar to graphite in terms of appearance, characteristics, and structure – it is black and flaky, conducts electricity, and has puckered sheets of connected atoms arranged in a hexagonal pattern.
Another allotrope of phosphorus is diphosphorus, which is composed of a phosphorus dimer as a structural unit and is extremely reactive in nature.
Phosphorus Oxygen Compounds
Phosphorus can be found in two common oxides: phosphorus(III) oxide (or tetraphosphorus hexaoxide), P4O6, and phosphorus(V) oxide (or tetraphosphorus decaoxide), P4O10. Phosphorus can also be found in phosphorus(III) oxide (or tetraphosphorus hexaoxide), P4O10..Phosphorus(III) oxide is a white crystalline solid with a garlic-like odour that can be found in small amounts in nature. The vapour produced by this plant is extremely harmful. It oxidises slowly in air and ignites when heated to 70 degrees Celsius, resulting in the formation of P4O10. Slowly dissolving phosphorus(III) oxide in cold water results in the formation of phosphorous acid, H3PO3.
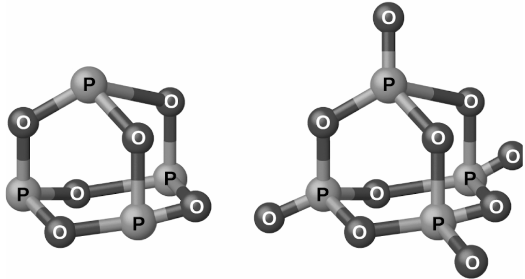
When phosphorus is burned in excess oxygen, it produces phosphorus(V) oxide (P4O10), a white powder that can be used as a catalyst. It has a very high enthalpy of formation (about 2984 kJ), is quite stable, and is a very weak oxidising agent. A hissing sound, heat, and orthophosphoric acid are all produced when P4O10 is dropped into water:
P4O10(s) + 6H2O(l) ⟶ 4H3PO4(aq)
In part due to its high affinity for water, phosphorus(V) oxide is a highly effective drying agent for gases and liquids, as well as an effective solvent for extracting water from numerous compounds.
Phosphorus Halogen Compounds
Direct reactions between phosphorus and halogens will result in the formation of trihalides (PX3) and pentahalides (PX5). Due to nitrogen’s inability to generate more than four bonds, trihalides are far more stable than the comparable nitrogen trihalides; nitrogen pentahalides, on the other hand, do not form.
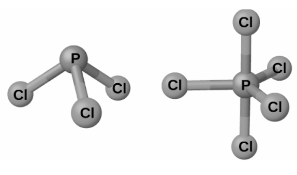
The chlorides PCl3 and PCl5 are the most abundant phosphorus halides on the earth’s surface. It is made by passing chlorine over a molten phosphorus solution to produce phosphorus trichloride, which is a white liquid. This off-white solid is made by reacting excess chlorine with phosphorus trichloride to form a compound known as phosphorus pentachloride. When heated, the pentachloride sublimes and creates an equilibrium with the trichloride and chlorine, which is known as a phase transition.
Both phosphorus chlorides, like most other nonmetal halides, react with an excess of water to produce hydrogen chloride and an oxyacid: PCl3 gives phosphorous acid H3PO3 and PCl5 yields phosphoric acid H3PO4 when exposed to an excess of water.
phosphorus pentahalides are Lewis acids due to the presence of vacant valence d orbitals in the phosphorus atom’s structure. It is easy for these compounds to react with halide ions (Lewis bases) and produce the anion PX6– . Unlike phosphorus pentafluoride, which is a molecular molecule in all states, X-ray studies have revealed that solid phosphorus pentachloride,[PCl4+ ][PCl6–], is an ionic compound, as is phosphorus pentabromide, [PBr4–][Br–], and phosphorus pentaiodide,[PI4+][I–], which are also ionic compounds.
Biological significance
All known forms of life necessitate the presence of inorganic phosphorus in the form of the phosphate PO43-. It has a significant role in biological molecules; for example, it is a structural component of DNA and RNA, which serve as structural frameworks. Consequently, phosphate salts are utilised in the production of fertilisers to aid in plant growth. Phosphate is also used by living cells to carry cellular energy in the form of adenosine triphosphate, which is a type of sugar (ATP). Adenosine triphosphate (ATP) is the energy source for nearly every cellular action that requires it. ATP is also required for phosphorylation, which is a critical process in the regulation and transmission of signals in cells. Phospholipids are the primary structural components of all cellular membranes. They are composed of a long alkyl chain that terminates in a phosphate group, which is the primary structural component of all cellular membranes. Calcium phosphate salts are beneficial in the hardening of bones.
CONCLUSION
A component of cell membranes and nucleic acids, phosphorus is also involved in a variety of biological activities, including bone mineralization, energy production and signalling through phosphorylation events in cells, as well as the regulation of acid-base balance.
Phosphorus, the eleventh most abundant element on the planet, is essential for the survival of all living beings. When it comes to humans, it is required for the production of DNA and cell membranes, as well as the formation of bone and teeth.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out