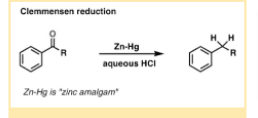
The Clemmensen Reduction Reaction uses concentrated hydrochloric acid (HCl) and zinc amalgam (Zn/Hg alloy) to convert aldehydes or ketones to alkanes. Erik Christian Clemmensen originally reported this in 1913 and it was given by his name.
Clemmensen Reduction Reaction
Clemmensen Reduction Reaction is a reaction that is used to convert aldehydes or ketones to alkanes. In a reduction reaction, oxygen atoms are removed from the molecule and electrons are gained.
The Clemmensen Reduction Reaction is extremely useful for reducing aryl-alkyl ketones produced via Friedel Crafts acylation. Friedel-Crafts acylation is used to produce acyl benzene. The Clemmensen reduction reaction is used to convert acyl benzene to alkyl benzene, as well as various ketones and aldehydes.
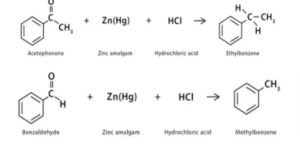
Examples
When acetophenone and benzaldehyde react with a reducing agent (Zn (Hg) & HCl) in the instances above, they produce ethylbenzene and methylbenzene, respectively.
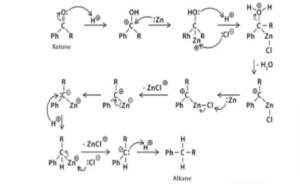
Clemmensen Reduction Reaction: Mechanism
When aldehydes and ketones react with zinc amalgam and strong hydrochloric acid, a hydrocarbon is generated due to deoxygenation, according to the Clemmensen reduction reaction mechanism. The reduction reaction happens on the zinc surface.
We still don’t have a solid method in place, but two suggestions have been offered, which are shown below:
Carbanionic Mechanism
Zinc is supposed to have a direct effect on protonated carbon in the carbanionic process.
Carbenoid Mechanism
The reactions on the metal surface of zinc decrease and take place on the surface of the zinc catalyst in the carbenoid mechanism. This is a radical procedure.
Intermediary of zinc is followed in the mechanism of Clemmensen reduction reactions. Deoxygenation of ketones and aldehydes results in the formation of hydrocarbons. In this reaction, the substrate must be a stable strong acid. The Clemmensen reduction is used in conjunction with the Wolff-Kishner reduction, which is carried out under very simple conditions.
Clemmensen Reduction is explained using the following reaction equations:
Wolf Kishner Reaction is Similar to Clemmensen Reaction in Following Ways:
- Wolf Kishner Alkanes are formed by heating carbonyl compounds with hydrazine and potassium hydroxide. Boiling solvents such as ethylene glycol or diethylene glycol are used.
- Clemmensen After heating, carbonyl compounds and hydrazine react to generate hydra zones, which are then converted to alkanes.
Wolf Kishner Reaction is different From Clemmensen Reaction as:
- In Wolf Kishner reaction Carbonyl molecules are converted to methylene compounds.
- Ketones or aldehydes are converted to alkanes in this reaction.
Application of Clemmensen Reduction
- Alkane can be made from alkenyl chloride (halide), which can then be used to make alkenyl halide.
- Carbonyl group to methyl group conversion
- Aromatics with unbranched side hydrocarbon chains and polycyclic aromatics are produced.
- Aliphatic and mixed aliphatic-aromatic carbonyl compounds are reduced.
- Alkyl benzene is formed by converting acyl benzene to alkyl benzene.
Wolff – Kishner Reduction
In nature, the Wolff-Kishner Reduction Mechanism is a type of organic chemical process. This reaction aids in the conversion of aldehydes and ketones to alkanes. The reaction begins with the creation of a hydrazone anion, which is followed by the release of a nitrogen atom, resulting in the formation of a carbanion.
- Kishner and Ludwig Wolff discovered the Wolff – Kishner Reduction Mechanism in 1912. It is a sort of organic chemical process in which aldehydes and ketones are reduced to alkanes. The reaction calls for the use of diethylene glycol as a solvent, which speeds up the reduction process.
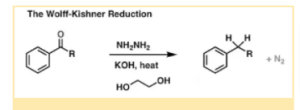
A hydrazone anion is formed in the beginning of the chemical process. The synthesis of the hydrazone anion results in the release of nitrogen atoms, which contribute to the development of carbanion through a chemical process. As a result, the next step includes the carbanion reacting with water, releasing its product in the form of a hydrocarbon.
So the double bond of carbon-oxygen can be changed into two single carbon-hydrogen bonds in strongly basic conditions, some aldehydes and ketones are stable in nature and can be easily reduced to alkanes. The use of a pre-formed hydrazone in the process can be advantageous since it reduces the time it takes for hydrazine to condense into hydrazone.
Conclusion
The Clemmensen Reduction Reaction uses concentrated hydrochloric acid (HCl) and zinc amalgam (Zn/Hg alloy) to convert aldehydes or ketones to alkanes.
Clemmensen Reduction Reaction is a reaction that is used to convert aldehydes or ketones to alkanes. In a reduction reaction, oxygen atoms are removed from the molecule and electrons are gained.
The Clemmensen Reduction Reaction is extremely useful for reducing aryl-alkyl ketones produced via Friedel Crafts acylation. Friedel-Crafts acylation is used to produce acyl benzene.
Wolf Kishner Reaction is different From Clemmensen Reaction as:
- In Wolf Kishner reaction Carbonyl molecules are converted to methylene compounds.
- Ketones or aldehydes are converted to alkanes in this reaction.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





