Phosphoric acid is a triprotic acid that exists as a thick liquid. It is also known as orthophosphoric acid. Both humans and laboratory animals’ skin, eyes, and other mucous membranes are irritated or corroded by it. Its salts, on the other hand, have a far reduced irritancy potential. When mice were exposed via inhalation, moderate toxicity was detected. Although phosphate salts have been shown to increase the action of recognised carcinogens, phosphoric acid is neither genotoxic nor carcinogenic. Irrigation or water flushing are commonly used to treat exposures. Phosphoric acid has attracted a lot of attention as a food ingredient for various cola drinks, but there’s a lot of debate over whether it’s safe. When it comes to pollution of the aquatic environment, the pH of the water is the most important factor to consider in terms of the effects on native flora and fauna. There is no evidence of quotes directly or bioaccumulation.
Phosphorus Halogen Compounds
Phosphorus reacts directly with halogens to create PX3 trihalides and PX5 pentahalides. The trihalides are substantially more stable than their nitrogen counterparts; nitrogen pentahalides do not develop because nitrogen cannot make more than four bonds.
The most important phosphorus halides are PX3 and PX5, which are both depicted in Figure. The colourless liquid phosphorus trichloride is made by passing chlorine over molten phosphorus. The off-white solid phosphorus pentachloride is made by oxidising phosphorus trichloride with sufficient chlorine. When heated, the pentachloride sublimes and creates an equilibrium with the trichloride and chlorine.
For example
reaction of white phosphorus with bromine
P4+6Br2 → 4PBr3
reaction of red phosphorus
4P+3Br2 → 2PBr3
reaction of phosphorus with excess chlorine
P4+10Cl2 → 4PCl5
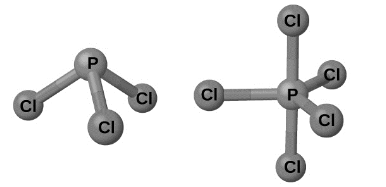
Both phosphorus chlorides, like most other nonmetal halides, react with an excess of water to produce hydrogen chloride and an oxyacid: PCl3 produces phosphorous acid H3PO3 while PCl5 produces phosphoric acid, H3PO4.
Because of the vacant valence d orbitals of phosphorus, pentahalides of phosphorus are Lewi’s acids. The anion PX6– is formed when these chemicals combine with halide ions (Lewis bases). While phosphorus pentafluoride is a molecular molecule in all states, X-ray studies reveal that solid phosphorus pentachloride, PCl4–[PCl6–], as well as phosphorus pentabromide, PBr4+[Br4–], and phosphorus pentaiodide, Pl4+[l–], is an ionic compound.
overview of phosphoric acid
Phosphorus is produced industrially by heating calcium phosphate, which is extracted from phosphate rock, with sand and coke:
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)→6CaSiO3(l)+10CO(g)+P4(g)
Phosphorus is distilled out of the furnace and either solidified or burned to generate P4O10 . P4O10 is
the starting point for numerous additional phosphorus compounds. Acids and phosphates are used in fertilisers and chemical manufacturing. Other applications include the production of specific alloys like ferrophosphorus and phosphor bronze. Phosphorus is necessary for the production of insecticides, matches, and some polymers. Phosphorus is a nonmetal that is active. Phosphorus is found in compounds in the oxidation states 3+, 3+, and 5+. In compounds containing phosphorus-phosphorus bonds, phosphorus has uncommon oxidation values for a group 15 element; examples include diphosphorus tetrahydride, H2P-PH2, and tetra phosphorus trisulfide, P4S3.
P4S3 is a component of the heads of strike-anywhere match
Properties and reactions
1s²2s²2p63s²3p³ represents the electron configuration of the phosphorus atom. With three half-filled orbitals capable of forming a single covalent bond and an additional lone pair of electrons, the outer shell arrangement resembles that of nitrogen. Phosphorus, like nitrogen, can have oxidation states of +3 or 3, depending on the electronegativity of the elements with which it reacts.
The main distinctions between nitrogen and phosphorus are that the latter has a lower electronegativity and larger atoms with more accessible outer d orbitals. Because of these factors, the similarities in nitrogen and phosphorus chemistry are mostly formal, masking the underlying, significant differences. The outer d orbitals of phosphorus allow the octet to expand, resulting in the +5 state, in which compounds form five genuine covalent bonds, a condition that nitrogen cannot achieve.
Principle compounds
Phosphorus is nearly entirely employed in the form of compounds, with oxidation states of +3, +5, and 3 being the most common. Phosphorus, unlike nitrogen and the other members of the family, prefers to be in the +5 state.
The chemical compound phosphine, also known as hydrogen phosphide (PH3), has a significant economic impact. The hydrolysis of a metal phosphide or the action of a strong base (or hot water) on white phosphorus yield this gaseous molecule. Phosphine is used as a fumigant, a doping agent for solid-state electronics components, and a precursor in the synthesis of numerous organic phosphorus compounds.
Phosphoric acid
Other mineral acids are more corrosive than phosphoric acid. Many materials, at least at low temperatures, show useful resistance to phosphoric acid. Corrosion rates are often accelerated by temperature and acid contaminants. Figure 24 depicts zirconium’s resistance to phosphoric acid attack at concentrations up to 55 percent and temperatures above the boiling point. The corrosion rate of zirconium increases with temperature above 55 percent phosphoric acid. Dilute acid at high temperatures would be the most interesting area for zirconium. The anodic polarisation curves of zirconium in phosphoric acid at near-boiling temperatures.
Conclusion
The page contains all of the critical information that a student needs to know about the phosphoric acid with halogen at a basic level, phosphoric acid with halogen compounds, properties and reactions, among other things. This is a vital piece of equipment for taking phosphoric acid with halogen.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




