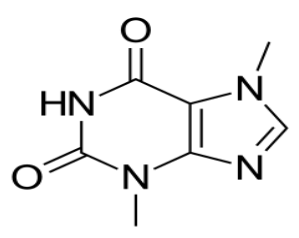
Caffeine is a methylxanthine alkaloid found in the seeds, nuts, or leaves of several plants native to South America and East Asia. It is structurally similar to adenosine and acts primarily as an adenosine receptor antagonist with psychotropic and anti-inflammatory properties.
Caffeine binds to adenosine receptors in the central nervous system (CNS) after ingestion, inhibiting adenosine binding. This inhibits adenosine’s downregulation of CNS activity, stimulating the activity of the brain’s medullary, vagal, vasomotor, and respiratory centres.
This agent also stimulates the CNS by promoting neurotransmitter release.Caffeine’s anti-inflammatory effects are due to nonselective competitive inhibition of phosphodiesterases (PDEs). PDE inhibition increases intracellular cyclic AMP (cAMP), activates protein kinase A, and inhibits leukotriene synthesis, resulting in decreased inflammation and innate immunity.
What is Caffeine?
Caffeine is a nitrogenous organic compound of the alkaloid group, which has significant physiological effects. Tea, coffee, guarana, maté, kola nuts, and cacao all contain caffeine.At atmospheric pressure, pure caffeine (trimethylxanthine) exists as a white powder or as silky needles that melt at 238 °C (460 °F) and sublime at 178 °C (352 °F). It dissolves readily in hot water and forms crystals of caffeine monohydrate upon cooling. Caffeine is less soluble in organic solvents than it is in hot water. It has no odour but a bitter taste.Caffeine is present in ground coffee in concentrations ranging from 0.75 to 1.5 percent by weight. As a result, the average cup of coffee contains approximately 100 mg (0.003 ounce) of caffeine. Tea’s caffeine content varies greatly depending on its strength, but it averages around 40 mg. A 12-ounce glass of carbonated cola beverage also contains about 40 mg (0.0014 ounce) of caffeine.
Chemical Reactivity of Caffeine with Water
The Caffeine dimer stability in the presence of surrounding water molecules that was investigated using ab initio calculations at the B3LYP/CBSB7 level of theory.
Dimers were created by arranging geometry optimized monomers in various relative orientations. At the same level of theory, these dimers were fully geometry optimised. The interaction of dimers and water was modelled in two ways. The geometry of dimers in aqueous medium was optimised using a polarizable conductor calculation model.
After that, the energy, enthalpy, and Gibbs energy of dimer formation were calculated. The thermodynamic parameters were discovered to be significantly different from experimental values. Caffeine dimer water interactions were also simulated by placing water molecules around the dimer at potential hydrogen bonding sites.
The number of water molecules was thus increased up to six.Water molecules were found to form hydrogen bonds with both caffeine molecules. The interplanar distance between caffeine and caffeine increased from 3.6 for isolated caffeine dimers (gas phase) to 3.9 for dimer water clusters. Caffeine dimer formation energy change (E) ranged from 5.2 to 6.0 kcal mol1 depending on their relative orientation in gas phase. For dimer formation in the gas phase, the average energy change, enthalpy change, and Gibbs energy change are 5.9, 3.9, and 8.4 kcal mol1, respectively.
For dimer–water clusters, the average formation energy change was 13.7 kcal mol1. The average standard Gibbs energy change of dimer–water cluster formation is 14.9 kcal mol1. In comparison to changes in formation energy, relatively large fluctuations in Gibbs energy were observed.
Synthesis of Caffeine:
- In general, the reaction of 1,3-dimethylurea with malonic ester or compounds with reactivity similar to malonic ester is used in laboratories to synthesise caffeine.
- Ethyl cyanoacetate is a chemical compound with ester and nitrile functional groups. It has the same reactivity with 1,3-dimethyl urea as the malonic ester. As a result, it is used in the production of caffeine.
- Caffeine is synthesised using 1,3-dimethyl urea and ethyl cyanoacetate, which results in the formation of substituted uracil compounds, which are then converted to theophylline and, finally, caffeine.
- Caffeine can also be synthesised by combining theobromine with methyl iodide and sodium methoxide.
Formula of Caffeine:
Caffeine is also known by the IUPAC name 1, 3, 7-Trimethylpurine-2,6-dione. It is an organic compound on the WHO’s list of the World’s Most Essential Medicines. It is also a popular stimulant and is a component of the widely consumed beverage coffee.
Caffeine’s chemical formula is as follows: C8H10N4O2
Caffeine can be found in 16 different species, the most common of which are coffee and tea plants. It’s also found in yerba mate leaves and guarana seeds.
Conclusion
Caffeine is a methylxanthine alkaloid found in the seeds, nuts, or leaves of several plants native to South America and East Asia. Caffeine is a nitrogenous organic compound of the alkaloid group, which has significant physiological effects. Caffeine is less soluble in organic solvents than it is in hot water. In general, the reaction of 1,3-dimethylurea with malonic ester or compounds with reactivity similar to malonic ester is used in laboratories to synthesise caffeine. Caffeine is synthesised using 1,3-dimethyl urea and ethyl cyanoacetate, which results in the formation of substituted uracil compounds, which are then converted to theophylline and, finally, caffeine.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out