Phenols are aromatic compounds that have a hydroxyl group directly attached to a benzene ring. They are also called carbolic acid. Phenols can be classified as mono, di, and trihydric phenols on the basis of a number of hydroxyl groups attached to the aromatic ring.
The chemical properties of phenol are given below:
- Electrophilic substitution reactions
- Halogenation Reaction
- Kolbe’s Reaction
- Reimer-Tiemann Reaction
- Reaction with Zinc Dust
- Oxidation of Phenol
Chemical Properties of Phenol
The chemical reactions of phenols can be classified under two headings – one is involving the cleavage of the O–H bond and the other one involves the cleavage of the C–O bond in it.
Reactions involving O–H bond cleavage:
- With metals:
Phenols react with metals such as Na, K, Al, etc., to form phenoxide with the release of the hydrogen gas. Apart from active metals, phenol gives sodium phenoxide on reaction with aqueous NaOH(sodium hydroxide) too, by releasing a water molecule.
Phenols are Bronsted acids, so they donate a proton to a stronger base like NaOH.
The acidity of phenols is due to the release of H+ ions from the hydroxyl group connected to a phenol. The reason behind the release of a proton is that the OH group is involved in resonance, and therefore, the oxygen gets a partial positive charge in it. This enables the H+ ion to move out easily from the compound, thereby making phenols a Bronsted acid. Also, phenols are stronger acids than their counterparts, alcohols, due to the fact that the phenoxide ion is stabilized by resonance. This makes the removal of H+ ions easier.
- Formation of Esters (Esterification)
Phenols react with carboxylic acids, acid anhydrides, and acid chlorides to form the compound esters. The esterification reaction is always carried out in the presence of sulphuric acid. The esterification reaction is reversible. The water molecule formed during the reaction is immediately removed to facilitate the completion of the reaction.
Esterification reaction with acid chloride takes place in the presence of pyridine in order to neutralize the HCl formed during the esterification reaction.
- Acetylation
The reaction of phenols with an acid anhydride and the introduction of acetyl (CH3CO–) are called acetylation, and the product obtained is called acetylsalicylic acid or aspirin.
- Electrophilic Aromatic Substitution
The –OH group in the phenol activates the benzene ring and pushes it towards the electrophilic substitution. Also, the substituents or the incoming groups are directed by the OH group towards the ortho and para positions of the benzene ring. The reason behind this is that the resonance effect of the ring structure makes these positions electron-rich in the whole benzene.
- Nitration Reaction
The Addition of an –NO2 group to the benzene ring is called nitration. Phenol reacts with dilute nitric acid at 298K to yield ortho and para nitrophenols.
The ortho and para nitrophenols obtained can be separated using the steam distillation method. The intramolecular hydrogen bonding in ortho nitrophenol makes it volatile. On the other hand, para nitrophenol is less volatile because it is bound in intermolecular hydrogen bonding.
The phenol yields 2,4,6 trinitrophenol or picric acid when treated with concentrated nitric acid. The yield is poor, Picric acid is a stronger acid than phenol because of the three electron-withdrawing nitro-groups in the benzene ring.
- Halogenation Reaction
The Phenols react with bromine to yield different kinds of products under different experimental conditions.
The phenol reacts with bromine in low polarity solvents, such as chloroform or CS2, at low temperatures to form mono bromophenols. The reason is that the non-polar or low-polar solvents can only activate the benzene ring in the 1 and 4 positions. Hence, only mono-substituted products are formed.
The presence of a highly activating group, –OH in phenol, makes polarization of the bromine molecule much easier.
The Phenol forms 2,4,6 tribromophenol on reaction with bromine water. The product formed is a white precipitate.
- Kolbe’s Reaction
The Phenol reacts with sodium hydroxide and carbon dioxide to form sodium salicylate. It is acidified to yield 2-hydroxy benzoic acid or known as salicylic acid. The phenoxide ion formed with NaOH has higher reactivity than the phenol towards aromatic, electrophilic substitution, and thus, acidifies to produce salicylic acid.
- Reimer-Tiemann Reaction
The Phenol, in reaction with chloroform (CHCl3), in the presence of NaOH, forms an aryl aldehyde compound. A –CHO group is introduced into the benzene ring, this reaction is known as the Reimer-Tiemann reaction. The electrophile formed in this reaction (:CCl2) is called the dichlorocarbene. An intermediate, substituted benzyl chloride is formed, which is then hydrolyzed into salicylaldehyde, the product, in the presence of an alkali.
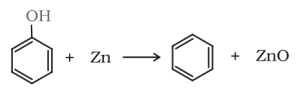
- Reaction with Zinc Dust
The Phenol, on heating with zinc dust, gets converted into benzene. The reaction is shown below:
- Oxidation of Phenol
The Phenol, on oxidation with chromic acid, forms a conjugated diketone called benzoquinone. In the presence of air, the oxidation reaction of phenols produces a dark-coloured mixture containing quinones.
Conclusion:
The chemical properties of phenol can be defined by the compounds it combines in different ways. Above we have discussed the definition of phenols. We have even talked about carbolic acid and focused more on the chemical properties of Phenol. We have discussed each of the chemical properties of phenols in detail. The chemical properties of phenol are electrophilic substitution reaction, halogenation reaction, Kolbe’s reaction, Reimer- Tiemann Reaction, reaction with zinc dust, and oxidation of phenols. Talking about the oxidation of phenol we have seen that on oxidation of phenol with chromic acid, forms a conjugated diketone called benzoquinone. The other property would be when phenol heated with zinc dust gets converted into benzene. Hence, we have discussed each detail of the chemical properties of phenol.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




