The term “neutral atom” refers to an atom that has the same number of protons as it does electrons. Essentially, every negative charge has an equal and opposite positive charge to counterbalance it. The number of electrons may, however, exceed the number of protons in some cases or vice versa, causing an imbalance in the charge of the atom, which results in the formation of an ion. Physicist and chemist Michael Faraday of the United Kingdom was the first to use the term ‘ion’ in 1834, following a suggestion made by English Polymath and Scientist William Whewell. These species, according to Michael Faraday, are those that move from one electrode to another through an aqueous medium. Faraday and Whewell are also credited with the invention of the terms ‘anion’ and ‘cation.'” After discovering that solid crystalline salts dissociate when dissolved, Swedish scientist Svante Arrhenius published an explanation in 1884. The Nobel Prize in Chemistry was awarded to him in 1903 for this achievement.
What are Anions?
Anions are ions that have a negative charge on their atoms or molecules. Some examples of a O2 CN, OH, ClO-2, CN2, OH-, CN-,OH- and Cl- etc. When an atom gains electron/s in order to achieve stability, it also gains a negative charge as a result of the fact that it has more electrons than protons in its nucleus. An anion is a species that is negatively charged, and it is found in nature. Faraday and Whewell were the first to use the term. It is derived from the Greek word ἄνω ἰόv (anion), which literally translates as ‘going up.
What are Cations?
Cations are ions that have a positive charge on their atoms or molecules. In addition to sodium and potassium ions, cations include aluminum and cerium ions. Examples of cations include sodium and potassium ions, aluminum and cerium ions, aluminum and cerium ions, aluminum and cerium ions, and aluminum and cerium ions. When an atom loses one or more electrons, it gains a positive charge as a result of the fact that it has fewer electrons than protons in its nucleus. The positively charged species is referred to as a cation in this case. Faraday and Whewell were the first to use the term. It is derived from the Greek words (káto) v (kation), which literally translates as ‘going down.’ Keep in mind that the sign for cation is the plus sign (‘+’), and that it is placed after the symbol rather than before it. Based on the number of electrons lost, a potassium atom is considered monovalent because it only loses one electron and thus has a positive charge of one. Magnesium, on the other hand, is considered divalent because it loses two or more electrons from its outermost orbit in order to form a stable octet. The cation name is defined as the name of the elemental element.
Diagram of Cation And Anions
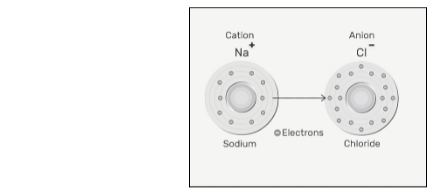
Causes anions and cations to form ionic bonds
When two cations and anions are joined together, this is known as ionic bonding. It is formed when an atom, usually a metal, loses one or more electrons and changes into a positive ion, also known as cation, and thus forms a bond with itself. One or more electron(s) can be acquired by another atom, typically a non-metal, allowing the latter to become known as a negative ion, also known as an anion.
Sodium fluoride, NaF, is formed when two sodium atoms come together with a single fluorine atom, which is an example of an ionic bond. A single valence electron is lost by the sodium atom and transferred to the fluorine atom, which has just enough space to accept it in this reaction. By virtue of electrostatic forces, the ions produced have opposing charges and are drawn to one another.
Ionic compounds form lattices at the macroscopic scale, are crystalline solids under normal conditions, and have extremely high melting points when heated. In water, most of these solids dissolve and become electrically conductive, which makes them useful in electronics. These substances are referred to as electrolytes because of their ability to conduct electricity in solution. NaCl (table salt) is a good example of this type of compound because it contains sodium chloride.
Conclusion
You should keep in mind that cations are positively charged ions, which means they have lost one or more electrons and thus contain a greater number of protons than electrons. It is known as ionization. Anions are negatively charged because they have gained one or more electrons and, as a result, have more electrons than protons in their ionisation.Chemists create ions from atoms and electrons that have gained or lost weight as a result of one or more valence electrons having been removed or added, respectively, to produce positive or negative charges. Ions are formed when one or more electrons are removed or added from an atom or electron, respectively, to produce either a positive or a negative charge.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




