The study of chemistry focuses on the formation of atoms by the aggregation of subatomic particles. Additionally, it is concerned with the way atoms form molecules. The atomic nucleus is surrounded by electrons in regions known as orbitals. There are only so many electrons that can fit in each orbital shell. This process continues until the next orbital shell out from the nucleus is also full, as new electrons accumulate in the closest orbital shell. The collection of electrons in ever-widening orbital shells continues as larger atoms have more electrons than smaller atoms. A molecule is formed when two atoms combine and their electrons mix into each other’s orbital shells. The molecule, like the atom, begins the formation of bonds at the nearest available opening in an orbital shell and works its way outwards. The chemical bond is a long-lasting attraction that permits chemical compounds to develop between atoms, ions, or molecules. Ionic bonds are created by the electrostatic attraction of ions with opposing charges, as opposed to covalent bonds, which are formed by the exchange of electrons. Chemical bonds can have a wide range of strength when it comes to chemistry.
Ionic and covalent bonds have been observed in the laboratory. A number of bond parameters, including length, angle, order, and energy, can be used to describe covalent bonds in this context (also known as bond enthalpy). Using these bond parameters, researchers can learn more about a chemical compound’s stability and bond strength. To be stable, many atoms must come together. Bond formation results in such a combination. Consequently, each bond has a characteristic associated with it.
Characteristics Of Covalent Bond
It is possible for atoms to share more than one pair of electrons in order to satisfy the normal valence of each atom. Covalent bonds have a number of characteristics, including:
No new electrons are created as a result of covalent bonding. It’s just the two of them.
Atomic bonds are extremely strong chemical ties.
- The energy of a covalent bond is approximately 80 kilocalories per mole (kcal/mol) on average.
- After a covalent bond is formed, it is extremely rare for it to break on its own.
- This type of bond is directional in that it shows the relative positions of the bonds between the atoms that are bonded to each other.
- The melting and boiling points of most covalent compounds are relatively low.
- Enthalpy values for compounds with covalent bonds tend to be lower.
- Because there are no free electrons in covalent compounds, they do not conduct electricity.
Compounds with a covalent structure are insoluble in water.
Bond Length
The distance between the nuclei of two chemically bonded atoms in a molecule is measured in bond length. The sum of the covalent radii of the two bonded atoms is approximately equal to this value. As the number of bonds increases, the length of the bonds decreases. This means that the bonds are stronger and more stable when the bond order is increased. These powerful forces of attraction lead to short bonds.
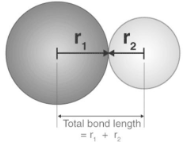
It is shown above how the total covalent radius sum of the atoms involved can be used to calculate the bond length. The following methods can be used to measure this bond parameter:
- Spectroscopy by rotating the sample.
- Diffraction of X-rays.
- Diffraction of neutrons.
It is common for bonded atoms to absorb thermal energy from their surroundings and vibrate constantly. The length of the bond changes as a result of the vibrations. The length of a covalent bond represents the average distance between the nuclei of the participating atoms, so it is important to keep this in mind.
Bond Angle
The angle formed between two covalent bonds that originate from the same atom can be defined as the bond angle. An illustration of the water molecule’s bond angle (104.50C) follows.
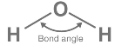
A bond angle is the angle between two adjacent covalent bonds. An understanding of a compound’s molecular geometry can be gained from knowing this bond parameter.
Bond Order
Bond order is the total number of electron pairs that can be covalently bonded between two atoms. Calculating it can be done by tracing out its Lewis structure and counting all of its pairs of electrons.
Examples :
Carbon-hydrogen bonds in C2H2 (ethyne/acetylene) have a bond order of 1, while carbon-carbon bonds have a bond order of 3.
An O2 molecule has an oxygen-oxygen bond order of 2.
Carbon monoxide has a bond order of 3 as shown in the Lewis structure below, which illustrates the carbon-oxygen bond.

Resonance aids the nitrate ion’s retention in this circumstance. The nitrogen-oxygen bond has a bond order of 4/3 or 1.33. The number of bonds is calculated by dividing the total number of nitrogen-oxygen bonds (4) by the total number of covalently bonded nitrogen-oxygen groups (3).
Bond Energy
To figure out the strength of a chemical bond, you look at how much “bond energy” the bond has. It is the amount of energy it takes to break all the covalent bonds in one mole of a chemical compound of a certain type (which is in its gaseous state).
It’s important to note that bond energy is not the same as bond dissociation energy, but both are important to know. When a bond is broken by homolytic cleavage, the enthalpy changes. This is the average of the bond dissociation enthalpy of all bonds of a certain type in the molecule.
Conclusion
The study of chemistry focuses on the formation of atoms by the aggregation of subatomic particles. Additionally, it is concerned with the way atoms form molecules. The atomic nucleus is surrounded by electrons in regions known as orbitals.Bond order is the total number of electron pairs that can be covalently bonded between two atoms. Calculating it can be done by tracing out its Lewis structure and counting all of its pairs of electrons.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




