The ionisation energy formula is the subject of this article. Ionization energy is the least amount of energy needed to remove the most loosely attached electron, the valence electron, from an isolated neutral gaseous atom or molecule. This energy was previously known as the ionisation potential, but that term is no longer in use.
Ionization Energy
Ionisation energy is a measure of how difficult it is to eliminate an electron from an atom or ion, or how likely an atom or ion is to yield an electron. The loss of 1 electron occurs in the chemical species’ ground state.
Ionization energy can either be adiabatic ionisation energy or vertical ionisation energy, depending on the ionisation of the molecule, which results in the changes in molecular geometry.
This approach can also be used to control the strength of chemical bonding.
Unit of the Ionization Energy
The ionisation energy is determined in electron-volts or kJ/mol units.
1 electron volt (eV) per atom is equal to 1.602×10-19 J per atom
1 electron volt (eV) per mole is equal to 1.602×10-19 ×6.022×1023 J/mol
1 electron volt (eV) per mole is equal to 196472 J/mol = 96.472 kJ/mol
Successive Ionization Enthalpies
If a gaseous atom needs to lose more than 1 electron, then they could be removed one at a time, rather than simultaneously. The term for this is successive ionisation enthalpy.
The number of electrons in the cation falls by one if the gaseous atom loses 1 electron to produce a monovalent cation, and the rest electrons are held by the cation’s nucleus with greater force. As a result, the 2nd electron cannot be removed with the same energy, necessitating the use of more energy to generate a divalent cation. The energy necessary to remove the 3rd electron, on the other hand, is projected to be significantly higher.
Ionization
Ionization is the process of removing an electron from an orbit and transferring it to the exterior of the atom. Ionization energy is equal to the difference in energy between the energy of the electron in the starting orbit and energy of the electron outside of the atom, because each orbit has a characteristic energy (in infinite orbit from nucleus).
The energy E is given as;
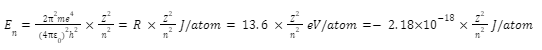
n = orbit
Z = atomic number
Trends of Ionization Energy in Periodic Table
An electron’s ionisation energy rises with the atomic number of atom and falls with higher energy orbitals. The ionisation energy increases as we move through the periodic table from left to right due to the decreasing atomic radius.
The ionisation energy drops as we proceed from top to bottom. This is owing to the fact that as we progress down the group, the elements have more electron shells. Furthermore, the electrons are separated from the nucleus’s attractive attractions by a wider distance.
Factors Affecting Ionization Enthalpy
Atomic size
The number of electron shells grows as the atomic scale increases. As a result, the force which binds electrons to the nucleus weakens. As the atomic size increases, the ionisation enthalpy decreases.
Nuclear Charge
The attraction of the +ve charge in nucleus of an atom to the electrons rises as the magnitude of positive charge increases. This indicates that more energy is required to remove the electrons that are concentrated in valence shell of gaseous atom. As a result, as the amount of nuclear charge grows, so does the ionisation enthalpy.
Screening or shielding effect
The screening effect grows in proportion to the number of electrons in inner shells. The effective nuclear charge falls as the screening effect increases. As a result, the nucleus’s force of attraction for valence shell electrons reduces, lowering the ionisation enthalpy. In simple terms, the ionisation enthalpy reduces as the shielding or screening effect of inner electrons increases.
Penetration effect of the electron
The penetration effect of the electron also influences the ionisation enthalpy, that is, the higher the possibility of finding the electron near to the nucleus, the stronger the electron’s attraction to the nucleus. As a result, removing an electron from an atom is tough. The order in which different sorts of electrons penetrate is s>p>d>f. The higher the penetration of electrons towards the atom’s nucleus, the higher the ionisation enthalpy (energy).
Ionization Energy Formula
IE=E-En
IE = Ionization Energy
n = number of states
Conclusion
You learned about the meaning of ionisation energy, successive ionisation enthalpies, it’s units, and how ionisation is affected by many parameters such as atomic radius, screening effect, and more.
Ionization energy is defined as the effort or difficulty involved in removing an electron from an atom or ion. Ionization energy can also be described as an atom’s or ion’s tendency to give up an electron.
The order of penetration of electrons is s>p>d>f.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




