Two or more chemicals can be mixed together in a solution, which can be either gaseous, liquid or solid. As the dissolving process heats up or cools down, so does the enthalpy change of solution (at constant pressure). Positive (endothermic) or negative enthalpy (ΔHSolution) can describe the state of a solution (exothermic). Think of an imaginary three-step process taking place between two components when you are thinking about the solution’s enthalpy.
Enthalpy of Solution
The enthalpy change associated with the dissolving of a substance in a solvent at constant pressure, resulting in infinite dilution, is known as the enthalpy of solution, enthalpy of dissolution, or heat of solution.
At constant temperature, kJ/mol is the most used unit for expressing solution enthalpy. There are three components to the energy change: the endothermic breakdown of bonds between the solute and the solvent, and the development of attractions between the solute and the solvent. The enthalpy of mixing in a perfect solution is zero. Extra molecular weight is the problem with non-ideal solutions.
Exothermic dissolution is common in the majority of gasses. To put it another way, the release of energy as heat when a gas combines with a liquid solvent causes the solution and its surroundings to heat up.
The solution’s temperature progressively drops to match the surrounding environment. As the temperature drops, the gas-solution equilibrium shifts in favor of the gas in solution, according to Le Châtelier’s principle (decreasing the temperature increases the solubility of a gas).
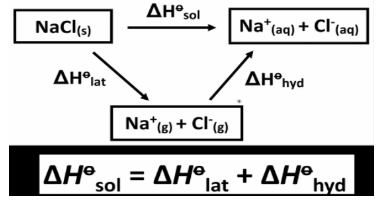
Heat Of A Solution
The heat solution is the difference in the enthalpy of the substance that is being dissolved in a solvent at the same pressure, which leads to infinite dilution. There is a unit for enthalpy called KJ/mol.
This happens when the solute is mixed with the solvent. When a solid or gas is mixed with the solvent, the heat is taken in. People call this type of process “heat dissolution” or “heat solution.” A calorimeter is used to measure the amount of heat in the solution.
There are three steps to solvation: breaking bonds between solute molecules, breaking intermolecular bonds between solvent molecules, and making new bonds between solute and solvent. It takes energy to get through the first two steps, and it takes it out at the end of the process.
Because of how much energy it takes to break bonds and how much is released when solutes and solvents form bonds, the overall heat of solution can be endothermic or exothermic.
Three Step Process Of Dissolution
- The heat of solution can be thought of as the sum of the enthalpy changes that occur in three different intermediate steps:
- The breaking of bonds inside a solute, for example, the electrostatic attraction between two ions (endothermic)
- The disintegration of intermolecular attractive interactions within the solvent, such as hydrogen bonds, in the presence of a solvent (endothermic)
- In solution, the creation of new and attractive solute-solvent bonds (exothermic)
The total heat of solution, denoted by the symbol ΔH∘sol, is the sum of the values of the separate steps. The overall heat of solution can be either positive or negative, depending on the respective signs and magnitudes of each step, and thus either endothermic or exothermic, depending on the relative signs and magnitudes of each step. This is totally dependent on whether or not more energy was expended in breaking the solute-solute and solvent-solvent bonds, or whether or not more energy was released when solute-solvent bonds were generated in the first place.
If more energy is released in the process of bond formation than is consumed in the process of bond breaking, the total process is exothermic, and ∆Hsol is negative. If more energy is expended in breaking bonds than is released during the creation of solute-solvent bonds, the process is said to be endothermic, and the value of ∆Hsol is positive.
Conclusion
It’s called the enthalpy of solutions when two substances mix together. This is how much heat each substance takes or gives off when they mix. In this case, it can be either positive or negative. Endothermic reactions happen when there is a positive enthalpy in the solution. This means that heat is taken in and it feels cold to touch. Exothermic reactions happen when there is a negative enthalpy in a solution. This causes heat to be released and makes it feel hot to the touch.
In order to dissolve something, you need to heat up or cool down something. A lot of heat will be lost or gained if three separate processes don’t happen at the same time.
The first thing that needs to happen is for the solute, the substance that is being dissolved, to separate from the rest of the water. Perhaps you have some table salt, NaCl, in your pantry or kitchen cupboard. Sodium and chloride are attracted to each other, so NaCl is made up of sodium and chloride atoms that stick together. So, if you want to dissolve NaCl, you’ll need to use a certain amount of power.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




